Dellagiustaite: A Novel Natural Spinel Containing V2+
Abstract
:1. Introduction
2. Occurrence and Paragenesis
3. Mineral Description and Physical Properties
4. Chemical Data
5. X-ray Crystallography
6. Results and Discussion
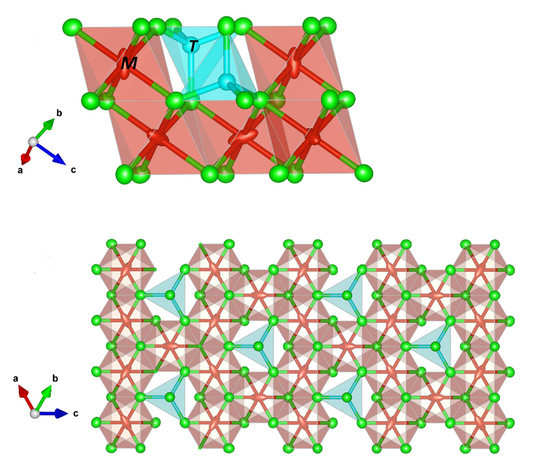
6.1. Description of the Crystal Structure
6.2. Conditions of Formation
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bosi, F.; Biagioni, C.; Pasero, M. Nomenclature and classification of the spinel supergroup. Eur. J. Mineral. 2018. [Google Scholar] [CrossRef]
- Bosi, F.; Andreozzi, G.B. Chromium influence on Mg-Al intracrystalline exchange in spinels and geothermometric implications. Am. Mineral. 2017, 102, 333–340. [Google Scholar] [CrossRef]
- Carpenter, M.A.; Salje, E.K.H. Thermodynamics of nonconvergent cation ordering in minerals: II. Spinels and the orthopyroxene solid solution. Am. Mineral. 1994, 79, 1068–1083. [Google Scholar]
- Kroll, H.; Schlenz, H.; Phillips, M.W. Thermodynamic modelling of non-convergent ordering in orthopyroxenes: A comparison of classical and Landau approaches. Phys. Chem. Miner. 1994, 21, 555–560. [Google Scholar] [CrossRef]
- Hazen, R.M.; Yang, H. Effects of cation substitution and order-disorder on P-V-T equations of state of cubic spinels. Am. Mineral. 1999, 84, 1956–1960. [Google Scholar] [CrossRef]
- Wong, J.; Lytle, F.W.; Messmer, R.P.; Maylotte, D.H. K-edge absorption spectra of selected vanadium compounds. Phys. Rev. B 1984, 30, 5596–5610. [Google Scholar] [CrossRef]
- Keppler, H. Crystal field spectra and geochemistry of transition metal ions in silicate melts and glasses. Am. Mineral. 1992, 77, 62–75. [Google Scholar]
- Sutton, S.R.; Karner, J.; Papike, J.; Delaney, J.S.; Shearer, C.; Newville, M.; Eng, P.; Rivers, M.; Dyar, M.D. Vanadium K edge XANES of synthetic and natural basaltic glasses and application to microscale oxygen barometry. Geochim. Cosmochim. Acta 2005, 69, 2333–2348. [Google Scholar] [CrossRef]
- Griffin, W.L.; Gain, S.E.M.; Adams, D.T.; Huang, J.-X.; Saunders, M.; Toledo, V.; Pearson, N.J.; O’Reilly, S.Y. First terrestrial occurrence of tistarite (Ti2O3): Ultra-low oxygen fugacity in the upper mantle beneath Mount Carmel, Israel. Geology 2016, 44, 815–818. [Google Scholar] [CrossRef]
- Xiong, Q.; Griffin, W.L.; Huang, J.X.; Gain, S.E.M.; Toledo, V.; Pearson, N.J.; O’Reilly, S.Y. Super-reduced mineral assemblages in “ophiolitic” chromitites and peridotites: The view from Mount Carmel. Eur. J. Mineral. 2017, 29, 557–570. [Google Scholar] [CrossRef]
- Griffin, W.L.; Huang, J.-X.; Thomassot, E.; Gain, S.E.M.; Toledo, V.; O’Reilly, S.Y. Super-reducing conditions in ancient and modern volcanic systems: Sources and behaviour of carbon-rich fluids in the lithospheric mantle. Mineral. Petrol. 2018, 112, 101–114. [Google Scholar] [CrossRef]
- Griffin, W.L.; Gain, S.E.M.; Huang, J.-X.; Saunders, M.; Shaw, J.; Toledo, V.; O’Reilly, S.Y. A terrestrial magmatic hibonite-grossite-vanadium assemblage: Desilication and extreme reduction in a volcanic plumbing system, Mt Carmel, Israel. Am. Mineral. 2019. [Google Scholar] [CrossRef]
- Otamendi, J.E.; Demichelis, A.H.; Tibaldi, A.M.; De La Rosa, J.D. Genesis of aluminous and intermediate granulites: A case study in the Eastern Sierras Pampeanas, Argentina. Lithos 2006, 89, 66–88. [Google Scholar] [CrossRef]
- Steenken, A.; Wemmer, K.; Martino, R.D.; López de Luchi, M.G.; Guereschi, A.; Siegesmund, S. Post-Pampean cooling and the uplift of the Sierras Pampeanas in the west of Córdoba (Central Argentina). Neues Jahrb. Geol. Palaontol. 2010, 256, 235–255. [Google Scholar] [CrossRef]
- Jacques, J.K. The heats of formation of cuspidine, 3CaO, 2SiO2, CaF2, and the mineral phase, 3CaO, 3Al2O3, CaF2. J. Chem. Soc. 1963, 9, 4297–4299. [Google Scholar] [CrossRef]
- Xia, Z.; Molokeev, M.S.; Oreshonkov, A.S.; Atuchin, V.V.; Liu, R.-S.; Dong, C. Crystal and local structure refinement in Ca2Al3O6F explored by X-ray diffraction and Raman spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 5952–5957. [Google Scholar] [CrossRef]
- Andersen, T.; Griffin, W.L.; O’Reilly, S.Y. Primary sulphide melt inclusions in mantle-derived megacrysts and pyroxenites. Lithos 1987, 20, 279–294. [Google Scholar] [CrossRef]
- Pouchou, J.L.; Pichoir, F. ‘PAP’ ϕ(ρZ) procedure for improved quantitative microanalysis. In Microbeam Analysis; Armstrong, J.T., Ed.; San Francisco Press: San Francisco, CA, USA, 1985; p. 104106. [Google Scholar]
- Petricek, V.; Dusek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General features. Z. Krist. Cryst. Mater. 2014, 229, 345–352. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- International Tables for Crystallography. Volume C: Mathematical, Physical and Chemical Tables; Wilson, A.J.C. (Ed.) Kluwer Academic: Dordrecht, The Netherland, 1992. [Google Scholar]
- Hartmann, H.; Maessing, W. Elektrolyse in Phosphatschmelzen. IV. Über die Elektrolyse von Vanadin-, Niob- und Tantaloxyd in Phosphatschmelzen. Z. Anorg. Allg. Chem. 1951, 266, 98–104. [Google Scholar] [CrossRef]
- Lavina, B.; Salviulo, G.; Della Giusta, A. Cation distribution and structure modelling of spinel solid solutions. Phys. Chem. Miner. 2002, 29, 10–18. [Google Scholar] [CrossRef]
- Rice, C.E.; Robinson, W.R. Structural changes in the solid solution (Ti1−xVx)2O3 as x varies from zero to one. J. Solid State Chem. 1977, 21, 145–154. [Google Scholar] [CrossRef]
- Urusov, V.S.; Serezhkin, V.N. Distortion of Vz+On coordination polyhedra and parameters of the bond valence model for VO bonds in inorganic crystals. Crystallogr. Rep. 2009, 54, 190–194. [Google Scholar] [CrossRef]
- Bosi, F.; Skogby, H.; Fregola, R.A.; Hålenius, U. Crystal chemistry of spinels in the system MgAl2O4-MgV2O4-Mg2VO4. Am. Mineral. 2016, 101, 580–586. [Google Scholar] [CrossRef]
- Matsuno, K.; Katsufuji, T.; Mori, S.; Moritomo, Y.; Machida, A.; Nishibori, E.; Takata, M.; Sakata, M.; Yamamoto, N.; Takagi, H. Charge Ordering in the Geometrically Frustrated Spinel AlV2O4. J. Phys. Soc. Jpn. 2001, 70, 1456–1459. [Google Scholar] [CrossRef]
- Kalavathi, S.; Raju, S.V.; Williams, Q.; Sahu, P.C.; Sastry, V.S.; Sahu, H.K. Pressure-induced frustration in charge ordered spinel AlV2O4. J. Phys. Condens. Matter 2013, 25, 292201. [Google Scholar] [CrossRef] [PubMed]
- Browne, A.J.; Kimber, S.A.J.; Attfield, J.P. Persistent three- and four-atom orbital molecules in the spinel AlV2O4. Phys. Rev. Mater. 2017, 1, 052003. [Google Scholar] [CrossRef]
- Talanov, M.V.; Shirokov, V.B.; Avakyan, L.A.; Talanov, V.M.; Borlakov, K.S. Vanadium clusters formation in geometrically frustrated spinel oxide AlV2O4. Acta Crystallogr. 2018, B74, 337–353. [Google Scholar] [CrossRef]
- Horibe, Y.; Kurushima, K.; Mori, S.; Asada, T.; Koyama, Y.; Shingu, M.; Katsufuji, T. Doping effect on the charge ordering in AlV2O4. Phys. Rev. B 2005, 71, 052411. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Di Pierro, S.; Gnos, E.; Grobety, B.H.; Armbruster, T.; Bernasconi, S.M.; Ulmer, P. Rock-forming moissanite (natural silicon carbide). Am. Mineral. 2003, 88, 1817–1821. [Google Scholar] [CrossRef]
- Curien, H.; Guillemin, C.; Orcel, J.; Steinberg, M. La hibonite, nouvelle espece minerale. Comptes Rendus Acad. Sci. 1956, 242, 2845–2847. (In French) [Google Scholar]
- Rakotondrazafy, M.A.; Moine, B.; Cuney, M. Mode of formation of hibonite (CaAl12O19) within the U-Th skarns from the granulites of S-E Madagascar. Contrib. Mineral. Petrol. 1996, 123, 190–201. [Google Scholar] [CrossRef]
- Ulianov, A.; Kalt, A. Mg–Al Sapphirine-and Ca–Al Hibonite-bearing Granulite Xenoliths from the Chyulu Hills Volcanic Field, Kenya. J. Petrol. 2006, 47, 901–927. [Google Scholar] [CrossRef]
- Konovalenko, S.I.; Ananyev, S.A.; Garmayeva, S.S. Rare and new minerals of the Tashelga-Maizaskaya zone of Gornaya Shoriya, their peculiarities and nature. J. Sib. Fed. Univ. Eng. Technol. 2012, 5, 301–310. [Google Scholar]
- Wild, M.; Milisenda, C.C. Hibonit aus Myanmar (Burma). Z. Dt. Gemmol. Ges. 2013, 62, 25–30. [Google Scholar]
- Hazen, R.M.; Grew, E.S.; Downs, R.T.; Golden, J.; Hystad, G. Mineral ecology: Chance and necessity in the mineral diversity of terrestrial planets. Can. Mineral. 2015, 53, 295–324. [Google Scholar] [CrossRef]
- Sruoga, P.; Ibanes, O.; Japas, M.S.; Urbina, N.E. El Morro caldera (33°10′ S, 66°24′ W), San Luis, Argentina: An exceptional case of fossil pre-collapse updoming. J. Volcanol. Geotherm. Res. 2017, 337, 81–97. [Google Scholar] [CrossRef]






| Constituent | Mean | Range | Probe Standard |
|---|---|---|---|
| MnO | 0.20 | 0.14–0.31 | Rhodonite |
| MgO | 1.82 | 1.17–2.24 | olivine |
| VO § | 32.38 | 29.73–33.15 | |
| V2O3 | 34.83 | 33.14–40.67 | V metal |
| Al2O3 | 29.55 | 24.47–31.18 | Grossular |
| Ti2O3 | 1.66 | 1.18–2.32 | Ilmenite |
| Total | 100.44 |
| Dgs * | Spinel | Spinel | Spinel | Grs *** | Grs | Hib | Hib **** | Ca2Al3 O6F | Pv ** | |
|---|---|---|---|---|---|---|---|---|---|---|
| Rock/Point | 12794B-2 | 12794B-7 | 12794B-10 | 12794B-11 | 12794B-19 | 12794B-5 | 12794B-4 | 12794B-6 | 12794B-8 | 12794B-12 |
| No. Oxy † | 4 | 4 | 4 | 4 | 7 | 7 | 19 | 19 | 7 | 3 |
| SiO2 | 0.02 | 0.04 | 0.00 | 0.04 | 0.14 | 0.06 | 0.03 | 0.09 | 1.16 | 3.29 |
| TiO2 | 0.73 | 0.00 | b.d.l. | b.d.l. | 0.01 | b.d.l. | 0.09 | 0.35 | 0.13 | 35.16 |
| Al2O3 | 32.63 | 39.61 | 38.47 | 50.51 | 77.35 | 77.35 | 90.12 | 85.24 | 54.35 | 13.46 |
| Ti2O3 | 0.66 | 1.37 | 0.81 | 0.72 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. |
| V2O3 | 34.88 | 28.01 | 32.43 | 20.93 | 0.52 | 0.74 | 2.41 | 6.12 | 0.27 | 4.86 |
| VO | 23.50 | 16.68 | 10.71 | 3.83 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.48 |
| CrO | 1.14 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.00 | b.d.l. | b.d.l. |
| MnO | 0.41 | 0.85 | 1.05 | 1.36 | 0.00 | b.d.l. | 0.05 | 0.01 | b.d.l. | b.d.l. |
| MgO | 7.23 | 12.99 | 16.64 | 22.87 | 0.08 | 0.03 | 0.18 | 0.63 | 0.05 | 0.05 |
| CaO | 0.57 | 0.80 | 0.91 | 0.68 | 21.65 | 21.84 | 8.50 | 8.41 | 36.33 | 38.74 |
| SrO | b.d.l. | 0.06 | b.d.l. | 0.08 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. |
| BaO | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.06 | b.d.l. | b.d.l. | b.d.l. | b.d.l. |
| Na2O~ | 0.17 | 0.07 | 0.05 | b.d.l. | b.d.l. | 0.07 | b.d.l. | 0.04 | 2.99 | 0.32 |
| K2O | 0.01 | 0.03 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.66 | 0.35 |
| La2O3 | 0.07 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.07 | b.d.l. | 0.00 |
| Ce2O3 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.12 | b.d.l. | 0.10 | b.d.l. |
| F | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 5.42 | b.d.l. |
| TOTAL | 102.02 | 100.51 | 101.09 | 101.03 | 99.76 | 100.16 | 101.51 | 100.97 | 101.46 | 96.38 |
| O=F | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.28 | 0.00 |
| TOTAL | 102.02 | 100.51 | 101.09 | 101.03 | 99.76 | 100.16 | 101.51 | 100.97 | 99.18 | 96.38 |
| Si | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.01 | 0.05 | 0.07 |
| Ti4+ | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.03 | 0.00 | 0.60 |
| Al | 1.12 | 1.32 | 1.26 | 1.54 | 3.96 | 3.96 | 11.73 | 11.31 | 2.99 | 0.36 |
| Ti3+ | 0.01 | 0.02 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Cr3+ | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| V3+ | 0.81 | 0.64 | 0.72 | 0.43 | 0.02 | 0.03 | 0.21 | 0.55 | 0.01 | 0.10 |
| V2+ | 0.61 | 0.42 | 0.27 | 0.09 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Cr2+ | 0.09 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Mn2+ | 0.01 | 0.02 | 0.02 | 0.03 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 |
| Ca | 0.02 | 0.02 | 0.03 | 0.02 | 1.01 | 1.02 | 1.01 | 1.01 | 1.82 | 0.94 |
| Mg | 0.31 | 0.55 | 0.69 | 0.88 | 0.01 | 0.00 | 0.03 | 0.11 | 0.00 | 0.00 |
| Na | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 | 0.27 | 0.01 |
| K | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 0.01 |
| Sum | 3.01 | 2.99 | 3.00 | 3.00 | 5.01 | 5.02 | 13.00 | 13.03 | 5.18 | 2.10 |
| Ba | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Sr | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| La | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ce3+ | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| F | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.80 | 0.00 |
| Mineral | Dellagiustaite | Hibonite | Grossite | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n. Points | 15 | Min. | Max. | Sd. | 3 | Min. | Max. | Sd. | 5 | Min. | Max. | Sd. |
| No. Oxy | 4 | 19 | 7 | |||||||||
| SiO2 | b.d.l. | b.d.l. | 0.05 | 0.00 | 0.27 | 0.12 | ||||||
| Al2O3 | 34.45 | 27.00 | 37.79 | 3.12 | 85.59 | 82.37 | 87.73 | 2.84 | 77.06 | 76.55 | 77.34 | 0.30 |
| TiO2 | b.d.l. | b.d.l. | b.d.l. | |||||||||
| Cr2O3 | 0.66 | 0.00 | 2.72 | 0.89 | b.d.l. | b.d.l. | ||||||
| MnO | 0.33 | 0.00 | 1.01 | 0.34 | b.d.l. | b.d.l. | ||||||
| V2O3 | 31.27 | 28.51 | 36.99 | 2.27 | 5.91 | 3.67 | 9.60 | 3.22 | 0.82 | 0.64 | 1.07 | 0.16 |
| VO | 21.42 | 13.65 | 30.26 | 4.83 | ||||||||
| MgO | 8.61 | 2.21 | 13.95 | 3.42 | 0.10 | 0.00 | 0.31 | 0.18 | b.d.l. | |||
| CaO | 0.66 | 0.28 | 0.83 | 0.15 | 8.18 | 7.91 | 8.60 | 0.37 | 22.05 | 21.80 | 22.16 | 0.15 |
| Na2O~ | b.d.l. | b.d.l. | 0.02 | 0.00 | 0.10 | 0.04 | ||||||
| K2O | b.d.l. | 0.22 | 0.00 | 0.65 | 0.37 | b.d.l. | ||||||
| TOTAL | 97.40 | 100.00 | 100.00 | |||||||||
| Si | 0.00 | 0.00 | 0.00 | |||||||||
| Ti4+ | 0.00 | 0.00 | 0.00 | |||||||||
| V3+ | 0.76 | 0.54 | 0.03 | |||||||||
| V2+ | 0.58 | 0.00 | 0.00 | |||||||||
| Al | 1.23 | 11.44 | 3.95 | |||||||||
| Cr3+ | 0.02 | 0.00 | 0.00 | |||||||||
| Mn2+ | 0.01 | 0.00 | 0.00 | |||||||||
| Ca | 0.02 | 0.99 | 1.03 | |||||||||
| Mg | 0.39 | 0.02 | 0.00 | |||||||||
| Na | 0.00 | 0.00 | 0.00 | |||||||||
| K | 0.00 | 0.03 | 0.00 | |||||||||
| Sum | 3.00 | 13.02 | 5.01 | |||||||||
| Comment | Krotite | Perovskite | K,Na,V-Rich Hibonite | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n. Points | 3 | Min. | Max. | Sd. | 1 | 9 | Min. | Max. | Sd. |
| No. Oxy | 4 | 3 | 19 | ||||||
| SiO2 | 0.68 | 0.55 | 0.77 | 0.12 | 0.32 | b.d.l. | |||
| Al2O3 | 63.14 | 62.64 | 63.47 | 0.43 | 3.52 | 85.10 | 83.27 | 87.60 | 1.49 |
| TiO2 | b.d.l. | 39.05 | b.d.l. | ||||||
| Cr2O3 | b.d.l. | 1.37 | b.d.l. | ||||||
| MnO | b.d.l. | b.d.l. | b.d.l. | ||||||
| V2O3 | 0.19 | 0.00 | 0.56 | 0.32 | 5.77 | 5.04 | 3.67 | 5.77 | 0.78 |
| VO | b.d.l. | ||||||||
| MgO | b.d.l. | b.d.l. | 2.24 | 0.68 | 4.45 | 1.27 | |||
| CaO | 35.72 | 35.56 | 36.02 | 0.26 | 39.22 | 2.39 | 0.00 | 6.47 | 2.58 |
| Na2O~ | b.d.l. | b.d.l. | 1.24 | 0.19 | 2.84 | 1.04 | |||
| K2O | b.d.l. | b.d.l. | 3.93 | 1.89 | 6.84 | 1.57 | |||
| TOTAL | 99.05 | 89.25 * | 99.94 | ||||||
| Si | 0.02 | 0.01 | 0.00 | ||||||
| Ti4+ | 0.00 | 0.70 | 0.00 | ||||||
| V3+ | 0.00 | 0.11 | 0.46 | ||||||
| V2+ | 0.00 | 0.00 | 0.00 | ||||||
| Al | 1.96 | 0.10 | 11.47 | ||||||
| Cr3+ | 0.00 | 0.02 | 0.01 | ||||||
| Mn2+ | 0.00 | 0.00 | 0.00 | ||||||
| Ca | 1.01 | 0.99 | 0.29 | ||||||
| Mg | 0.00 | 0.00 | 0.38 | ||||||
| Na | 0.01 | 0.00 | 0.28 | ||||||
| K | 0.00 | 0.00 | 0.57 | ||||||
| Sum | 2.98 | 1.93 ** | 12.61 | ||||||
| dobs | dcalc | I | h | k | l |
|---|---|---|---|---|---|
| 4.727 | 4.731 | 5 | 1 | 1 | 1 |
| 2.895 | 2.897 | 5 | 2 | 0 | 2 |
| 2.469 | 2.471 | 19 | 1 | 1 | 3 |
| 2.047 | 2.049 | 58 | 0 | 0 | 4 |
| 1.576 | 1.577 | 38 | 3 | 3 | 3 |
| 1 | 1 | 5 | |||
| 1.447 | 1.449 | 100 | 4 | 0 | 4 |
| 1.249 | 1.250 | 8 | 3 | 3 | 5 |
| 1.234 | 1.235 | 6 | 2 | 2 | 6 |
| 1.182 | 1.183 | 27 | 4 | 4 | 4 |
| 1.066 | 1.067 | 18 | 3 | 1 | 7 |
| 5 | 3 | 5 | |||
| 1.023 | 1.024 | 87 | 0 | 0 | 8 |
| 0.945 | 0.946 | 16 | 5 | 1 | 7 |
| 5 | 5 | 5 | |||
| 0.939 | 0.940 | 5 | 6 | 2 | 6 |
| 0.915 | 0.916 | 21 | 4 | 0 | 8 |
| 0.873 | 0.874 | 18 | 6 | 4 | 6 |
| 0.858 | 0.859 | 8 | 3 | 1 | 9 |
| 0.836 | 0.836 | 35 | 4 | 4 | 8 |
| 0.803 | 0.804 | 12 | 6 | 2 | 8 |
| 2 | 0 | 10 |
| Crystal Data | ||
| dellagiustaite | V-rich spinel | |
| Crystal size (mm3) | 0.149 × 0.137 × 0.095 | 0.120 × 0.100 × 0.060 |
| Cell setting, space group | Cubic, Fdm | Cubic, Fdm |
| a (Å) | 8.1950(1) | 8.1754(2) |
| V (Å3) | 550.36(1) | 546.42(2) |
| Z | 8 | 8 |
| Data Collection and Refinement | ||
| Radiation, wavelength (Å) | Mo Kα, λ = 0.71073 | Mo Kα, λ = 0.71073 |
| Temperature (K) | 293 | 293 |
| 2θmax (°) | 72.1 | 72.2 |
| Measured reflections | 3605 | 3579 |
| Unique reflections | 85 | 85 |
| Reflections with Fo > 4σ (Fo) | 81 | 77 |
| Rint | 0.0194 | 0.0337 |
| Rσ | 0.0055 | 0.0092 |
| Range of h, k, l | −13 ≤ h ≤ 13, −10 ≤ k ≤ 10, −12 ≤ l ≤ 12 | −13 ≤ h ≤ 13, −10 ≤ k ≤ 10, −13 ≤ l ≤ 13 |
| R (Fo > 4σ (Fo)) | 0.0140 | 0.0166 |
| R (all data) | 0.0151 | 0.0215 |
| wR (on F2) | 0.0339 | 0.0343 |
| GooF | 1.245 | 1.402 |
| Number of least-square parameters | 11 | 11 |
| Maximum and minimum residuals (e/Å3) | 0.29 (at 0.74 from M), −0.56 (at 0.68 from M) | 0.30 (at 0.83 from M), −0.27 (at 1.66 from O) |
| Site | Site Occ. | x/a | y/b | z/c | Ueq |
|---|---|---|---|---|---|
| Dellagiustaite | |||||
| T | Al3+ 0.96(2) V3+ 0.04(2) | 1/8 | 1/8 | 1/8 | 0.0099(4) |
| M | Al3+ 0.04(3) V3+ 0.96(3) | 1/2 | 1/2 | 1/2 | 0.0154(2) |
| O | O0 0.90(17) O= 0.10(17) | 0.25054(11) | 0.25054(11) | 0.25054(11) | 0.0108(5) |
| V-rich spinel | |||||
| T | Al3+ 0.97(2) V3+ 0.03(2) | 1/8 | 1/8 | 1/8 | 0.0101(3) |
| M | Al3+ 0.44(2) V3+ 0.56(2) | 1/2 | 1/2 | 1/2 | 0.0111(2) |
| O | O0 0.82(13) O= 0.18(13) | 0.25612(10) | 0.25612(10) | 0.25612(10) | 0.0129(5) |
| Dellagiustaite | V-Rich Spinel | |
|---|---|---|
| T–O × 4 | 1.782(2) | 1.8567(14) |
| M–O × 6 | 2.0445(9) | 1.9951(7) |
| Site | Site Occupancies (apfu) | s.s. (eps) | m.b.l. (Å) | ||
|---|---|---|---|---|---|
| Refined | Calculated | Refined | Calculated | ||
| Dellagiustaite | |||||
| T | 1 Al3+ | 13.4(2) | 13 | 1.782(2) | 1.774 |
| M | 0.88 V3+ + 0.91 V2+ + 0.09 Al3+ + 0.08 Mg + 0.03 Ti3+ + 0.01 Mn2+ | 22.51(3) | 21.78 | 2.045(5) | 2.038 |
| V-Rich Spinel | |||||
| T | 0.53 Al3+ + 0.47 Mg | 13.3(2) | 12.53 | 1.857(2) | 1.864 |
| M | 0.80 Al3+ + 0.64 V3+ +0.43 V2+ + 0.08 Mg + 0.03 Ti3+ + 0.02 Ca | 18.6(2) | 18.19 | 1.995(1) | 1.989 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cámara, F.; Bindi, L.; Pagano, A.; Pagano, R.; Gain, S.E.M.; Griffin, W.L. Dellagiustaite: A Novel Natural Spinel Containing V2+. Minerals 2019, 9, 4. https://doi.org/10.3390/min9010004
Cámara F, Bindi L, Pagano A, Pagano R, Gain SEM, Griffin WL. Dellagiustaite: A Novel Natural Spinel Containing V2+. Minerals. 2019; 9(1):4. https://doi.org/10.3390/min9010004
Chicago/Turabian StyleCámara, Fernando, Luca Bindi, Adriana Pagano, Renato Pagano, Sarah E. M. Gain, and William L. Griffin. 2019. "Dellagiustaite: A Novel Natural Spinel Containing V2+" Minerals 9, no. 1: 4. https://doi.org/10.3390/min9010004
APA StyleCámara, F., Bindi, L., Pagano, A., Pagano, R., Gain, S. E. M., & Griffin, W. L. (2019). Dellagiustaite: A Novel Natural Spinel Containing V2+. Minerals, 9(1), 4. https://doi.org/10.3390/min9010004