Utilization of Sodium Hexametaphosphate for Separating Scheelite from Calcite and Fluorite Using an Anionic–Nonionic Collector
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Experimental Procedures
2.3. Equilibrium Speciation Modelling
2.4. Zeta Potential Tests
2.5. FTIR Tests
2.6. XPS Tests
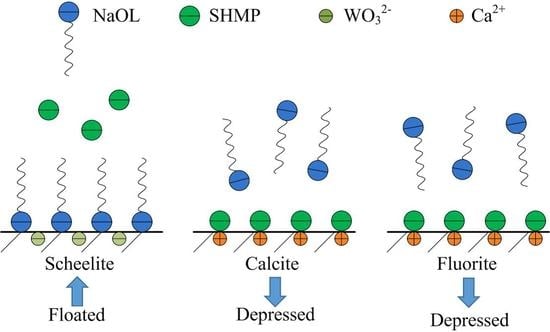
3. Results and Discussions
3.1. Flotation Experiments of Pure Mineral
3.2. Equilibrium Speciation Analysis
3.3. Zeta Potential Analysis
3.4. FTIR Analysis
3.5. XPS Analysis
3.6. Low-Grade Scheelite Ore Flotation Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shemi, A.; Magumise, A.; Ndlovu, S.; Sacks, N. Recycling of tungsten carbide scrap metal: A review of recycling methods and future prospects. Miner. Eng. 2018, 122, 195–205. [Google Scholar] [CrossRef]
- Jiao, F.; Dong, L.; Qin, W.; Liu, W.; Hu, C. Flotation separation of scheelite from calcite using pectin as depressant. Miner. Eng. 2019, 136, 120–128. [Google Scholar] [CrossRef]
- Yang, X. Beneficiation studies of tungsten ores—A review. Miner. Eng. 2018, 125, 111–119. [Google Scholar] [CrossRef]
- Kang, J.; Liu, Y.; Ahmed Khoso, S.; Hu, Y.; Sun, W.; Liu, R. Significant improvement in the scheelite heating flotation with sodium sulfide. Minerals 2018, 8, 587. [Google Scholar] [CrossRef]
- Kang, J.; Chen, C.; Sun, W.; Tang, H.; Yin, Z.; Liu, R.; Hu, Y.; Nguyen, A.V. A significant improvement of scheelite recovery using recycled flotation wastewater treated by hydrometallurgical waste acid. J. Clean. Prod. 2017, 151, 419–426. [Google Scholar] [CrossRef]
- Abdalla, M.A.M.; Peng, H.; Younus, H.A.; Wu, D.; Abusin, L.; Shao, H. Effect of synthesized mustard soap on the scheelite surface during flotation. Colloids Surf. A Physicochem. Eng. Asp. 2018, 548, 108–116. [Google Scholar] [CrossRef]
- Kupka, N.; Rudolph, M. Froth flotation of scheelite—A review. Int. J. Min. Sci. Technol. 2018, 28, 373–384. [Google Scholar] [CrossRef]
- Gao, Z.; Sun, W.; Hu, Y. New insights into the dodecylamine adsorption on scheelite and calcite: An adsorption model. Miner. Eng. 2015, 79, 54–61. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Z.; Gao, Y.; Hu, Y.; Sun, W. Flotation separation of scheelite from calcite using mixed cationic/anionic collectors. Miner. Eng. 2016, 98, 261–263. [Google Scholar] [CrossRef]
- Dong, L.; Jiao, F.; Qin, W.; Zhu, H.; Jia, W. Effect of acidified water glass on the flotation separation of scheelite from calcite using mixed cationic/anionic collectors. Appl. Surf. Sci. 2018, 444, 747–756. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, H.; Sun, W.; Hu, Y.; Qin, W.; Liu, R. Synergetic effect of the mixed anionic/non-ionic collectors in low temperature flotation of scheelite. Minerals 2017, 6, 87. [Google Scholar] [CrossRef]
- Zhu, H.; Qin, W.; Chen, C.; Liu, R. Interactions between sodium oleate and polyoxyethylene ether and the application in the low-temperature flotation of scheelite at 283 K. J. Surfact. Deterg. 2016, 19, 1289–1295. [Google Scholar] [CrossRef]
- Filippova, I.V.; Filippov, L.O.; Duverger, A.; Severov, V.V. Synergetic effect of a mixture of anionic and nonionic reagents: Ca mineral contrast separation by flotation at neutral pH. Miner. Eng. 2014, 66–68, 135–144. [Google Scholar] [CrossRef]
- Sis, H.; Chander, S. Improving froth characteristics and flotation recovery of phosphate ores with nonionic surfactants. Miner. Eng. 2003, 16, 587–595. [Google Scholar] [CrossRef]
- Kang, J.; Hu, Y.; Sun, W.; Liu, R.; Yin, Z.; Tang, H.; Meng, X.; Zhang, Q.; Liu, H. A significant improvement of scheelite flotation efficiency with etidronic acid. J. Clean. Prod. 2018, 180, 858–865. [Google Scholar] [CrossRef]
- Wei, Q.; Dong, L.; Jiao, F.; Qin, W. Use of citric acid and Fe (III) mixture as depressant in calcite flotation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123579. [Google Scholar] [CrossRef]
- Zhang, C.; Wei, S.; Hu, Y.; Tang, H.; Gao, J.; Yin, Z.; Guan, Q. Selective adsorption of tannic acid on calcite and implications for separation of fluorite minerals. J. Colloid Interface Sci. 2018, 512, 55–63. [Google Scholar] [CrossRef]
- Dong, L.; Jiao, F.; Qin, W.; Zhu, H.; Jia, W. New insights into the carboxymethyl cellulose adsorption on scheelite and calcite: Adsorption mechanism, AFM imaging and adsorption model. Appl. Surf. Sci. 2019, 463, 105–114. [Google Scholar] [CrossRef]
- Feng, B.; Luo, X.; Wang, J.; Wang, P. The flotation separation of scheelite from calcite using acidified sodium silicate as depressant. Miner. Eng. 2015, 80, 45–49. [Google Scholar]
- Wei, Z.; Hu, Y.; Han, H.; Sun, W.; Wang, R.; Wang, J. Selective flotation of scheelite from calcite using Al-Na2SiO3 polymer as depressant and Pb-BHA complexes as collector. Miner. Eng. 2018, 120, 29–34. [Google Scholar] [CrossRef]
- Liu, C.; Feng, Q.; Zhang, G.; Chen, W.; Chen, Y. Effect of depressants in the selective flotation of scheelite and calcite using oxidized paraffin soap as collector. Int. J. Miner. Process. 2016, 157, 210–215. [Google Scholar] [CrossRef]
- Lü, Y.; Li, C. Selective flotation of scheelite from calcium minerals with sodium oleate as a collector and phosphates as modifiers. I. Selective flotation of scheelite. Int. J. Miner. Process. 1983, 10, 205–218. [Google Scholar]
- Chen, W.; Feng, Q.; Zhang, G.; Yang, Q.; Zhang, C.; Xu, F. The flotation separation of scheelite from calcite and fluorite using dextran sulfate sodium as depressant. Int. J. Miner. Process. 2017, 169, 53–59. [Google Scholar] [CrossRef]
- Lu, J.; Sun, M.; Yuan, Z.; Qi, S.; Tong, Z.; Li, L.; Meng, Q. Innovative insight for sodium hexametaphosphate interaction with serpentine. Colloids Surf. A Physicochem. Eng. Asp. 2019, 560, 35–41. [Google Scholar] [CrossRef]
- Li, C.; Lü, Y. Selective flotation of scheelite from calcium minerals with sodium oleate as a collector and phosphates as modifiers. II. The mechanism of the interaction between phosphate modifiers and minerals. Int. J. Miner. Process. 1983, 10, 219–235. [Google Scholar]
- Choi, I.K.; Wen, W.W.; Smith, R.W. The effect of a long chain phosphate on the adsorption of collectors on kaolinite. Miner. Eng. 1993, 6, 1191–1197. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, Z.; Sun, W.; Yin, Z.; Wang, J.; Hu, Y. Adsorption of a novel reagent scheme on scheelite and calcite causing an effective flotation separation. J. Colloid Interface Sci. 2018, 512, 39–46. [Google Scholar] [CrossRef]
- Gao, Z.; Bai, D.; Sun, W.; Cao, X.; Hu, Y. Selective flotation of scheelite from calcite and fluorite using a collector mixture. Miner. Eng. 2015, 72, 23–26. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, Z.; Gao, H.; Zheng, R.; Jin, Y.; Niu, C. Flotation studies of fluorite and barite with sodium petroleum sulfonate and sodium hexametaphosphate. J. Mater. Res. Technol. 2019, 8, 1267–1273. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, M.; Feng, Q.; Long, T.; Ou, L.; Zhang, G. Effect of sodium hexametaphosphate on separation of serpentine from pyrite. Trans. Nonferr. Metal. Soc. 2011, 21, 208–213. [Google Scholar] [CrossRef]
- Li, Z.; Han, Y.; Li, Y.; Gao, P. Effect of serpentine and sodium hexametaphosphate on ascharite flotation. Trans. Nonferr. Metal. Soc. 2017, 27, 1841–1848. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Q.; Zhang, G.; Yang, Q. Investigations on flotation separation of scheelite from calcite by using a novel depressant: Sodium phytate. Miner. Eng. 2018, 126, 116–122. [Google Scholar] [CrossRef]
- Espiritu, E.R.L.; Naseri, S.; Waters, K.E. Surface chemistry and flotation behavior of dolomite, monazite and bastnäsite in the presence of benzohydroxamate, sodium oleate and phosphoric acid ester collectors. Colloids Surf. A Physicochem. Eng. Asp. 2018, 546, 254–265. [Google Scholar] [CrossRef]
- Azizi, D.; Larachi, F. Surface interactions and flotation behavior of calcite, dolomite and ankerite with alkyl hydroxamic acid bearing collector and sodium silicate. Colloids Surf. A Physicochem. Eng. Asp. 2018, 537, 126–138. [Google Scholar] [CrossRef]
- Deng, L.; Zhao, G.; Zhong, H.; Wang, S.; Liu, G. Investigation on the selectivity of n-((hydroxyamino)-alkyl) alkylamide surfactants for scheelite/calcite flotation separation. J. Ind. Eng. Chem. 2016, 33, 131–141. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, Y.; Sun, W.; Zhai, J.; Yin, Z.; Guan, Q. Effect of phytic acid on the surface properties of scheelite and fluorite for. Colloids Surf. A Physicochem. Eng. Asp. 2019, 537, 80–87. [Google Scholar] [CrossRef]
- Gao, J.; Sun, W.; Hu, Y.; Wang, L.; Liu, R.; Gao, Z.; Chen, P.; Tang, H.; Jiang, W.; Lyu, F. Propyl gallate a novel collector for flotation separation of fluorite from calcite. Chem. Eng. Sci. 2019, 193, 255–263. [Google Scholar] [CrossRef]













| Elements | O | F | Na | Mg | Al | Si | P | S | K | Ca | Mn | Fe | Mo | W |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Contents (%) | 35.25 | 4.46 | 0.51 | 1.81 | 2.45 | 18.34 | 0.69 | 0.81 | 1.64 | 21.91 | 1.08 | 10.94 | 0.02 | 0.09 |
| Elements | Atomic Contents Changes (%) | ||
|---|---|---|---|
| Scheelite | Calcite | Fluorite | |
| C | 2.16 | −2.23 | 4.34 |
| O | −1.47 | 3.45 | 6.72 |
| W | −0.83 | - | - |
| F | - | - | −8.41 |
| Ca | −0.37 | −3.13 | −4.78 |
| P | 0.51 | 1.91 | 2.13 |
| Minerals | Scheelite | Calcite | Fluorite | |||
|---|---|---|---|---|---|---|
| Elements | Ca2p1 | Ca2p3 | Ca2p1 | Ca2p3 | Ca2p1 | Ca2p3 |
| Before (eV) | 350.51 | 346.94 | 350.56 | 346.99 | 351.37 | 347.79 |
| After (eV) | 350.54 | 346.94 | 350.69 | 347.13 | 351.82 | 348.25 |
| Shifts (eV) | 0.03 | 0 | 0.13 | 0.14 | 0.45 | 0.46 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.; Hu, Y.; Sun, W.; Gao, Z.; Liu, R. Utilization of Sodium Hexametaphosphate for Separating Scheelite from Calcite and Fluorite Using an Anionic–Nonionic Collector. Minerals 2019, 9, 705. https://doi.org/10.3390/min9110705
Kang J, Hu Y, Sun W, Gao Z, Liu R. Utilization of Sodium Hexametaphosphate for Separating Scheelite from Calcite and Fluorite Using an Anionic–Nonionic Collector. Minerals. 2019; 9(11):705. https://doi.org/10.3390/min9110705
Chicago/Turabian StyleKang, Jianhua, Yuehua Hu, Wei Sun, Zhiyong Gao, and Runqing Liu. 2019. "Utilization of Sodium Hexametaphosphate for Separating Scheelite from Calcite and Fluorite Using an Anionic–Nonionic Collector" Minerals 9, no. 11: 705. https://doi.org/10.3390/min9110705
APA StyleKang, J., Hu, Y., Sun, W., Gao, Z., & Liu, R. (2019). Utilization of Sodium Hexametaphosphate for Separating Scheelite from Calcite and Fluorite Using an Anionic–Nonionic Collector. Minerals, 9(11), 705. https://doi.org/10.3390/min9110705