Organo-Modified Vermiculite: Preparation, Characterization, and Sorption of Arsenic Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
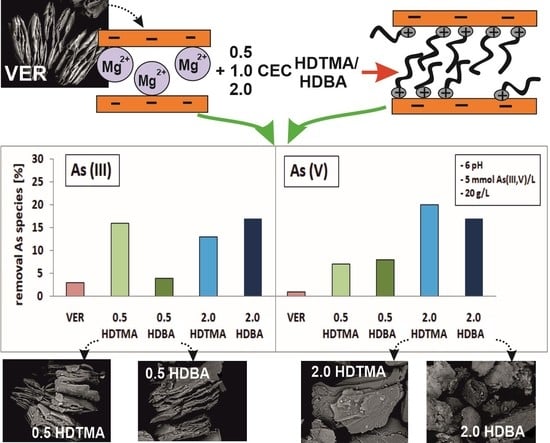
- HDTMA-modified VER at the concentrations of 0.5, 1.0, and 2.0 CEC: 0.5 HDTMA, 1.0 HDTMA, and 2.0 HDTMA, respectively.
- HDBA-modified VER at the concentrations of 0.5, 1.0, and 2.0 CEC: 0.5 HDBA, 1.0 HDBA, and 2.0 HDBA, respectively.
2.2. Sorption Experiments
- In the first stage, experiments were conducted using VER and all organo-sorbents, i.e., 0.5, 1.0, 2.0 HDTMA and 0.5, 1.0, 2.0 HDBA.
- In the second stage, experiments were conducted using VER, 0.5, 2.0 HDTMA, and 0.5, 2.0 HDBA.
2.3. Adsorption Models
2.4. Analytical Methods
3. Results
3.1. Modification of Vermiculite with HDTMA and HDBA
3.1.1. XRD
3.1.2. FTIR
3.1.3. N2-BET
3.1.4. DTA/TG
3.1.5. SEM
3.2. Sorption of As(III) and As(V) onto Modified and Unmodified Vermiculite
3.2.1. pH Effect
3.2.2. Initial Concentration Effect
3.2.3. Sorbent Dosage Effect
3.2.4. Adsorption Isotherms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bakshi, S.; Banik, C.; Rathke, S.J.; Laird, D.A. Arsenic sorption on zero-valent iron-biochar complexes. Water Res. 2018, 137, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Debure, M.; Tournassat, C.; Lerouge, C.; Made, B.; Robinet, J.C.; Fernandez, A.M.; Grangeon, S. Retention of arsenic, chromium and boron on an outcropping clay-rich rock formation (the Tegulines Clay, eastern France). Sci. Total Environ. 2018, 642, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.K.; Suzuki, K.T. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Vahidnia, A.; Van der Voet, G.B.; de Wolf, F.A. Arsenic neurotoxicity—A review. Hum. Exp. Toxicol. 2007, 26, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Paul, B. The global menace of arsenic and its conventional remediation—A critical review. Chemosphere 2016, 158, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Sowers, T.D.; Harrington, J.M.; Polizzotto, M.L.; Duckworth, O.W. Sorption of arsenic to biogenic iron (oxyhydr) oxides produced in circumneutral environments. Geochim. Cosmochim. Acta 2017, 198, 194–207. [Google Scholar] [CrossRef]
- The World Health Organization. Guideliness for drinking-water quality. WHO Chron. 2011, 38, 104–108. [Google Scholar]
- Elwakeel, K.Z.; Guibal, E. Arsenic(V) sorption using chitosan/Cu(OH)2 and chitosan/CuO composite sorbents. Carbohydr. Polym. 2015, 134, 190–204. [Google Scholar] [CrossRef]
- Ali, I. The Quest for Active Carbon Adsorbent Substitutes: Inexpensive Adsorbents for Toxic Metal Ions Removal from Wastewater. Sep. Purif. Rev. 2010, 39, 95–171. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, C.W.; Wang, Y.G.; Fan, W.H.; Luan, Z.K. Effects of ion concentration and natural organic matter on arsenic(V) removal by nanofiltration under different transmembrane pressures. J. Environ. Sci. 2013, 25, 302–307. [Google Scholar] [CrossRef]
- Wang, Y.X.; Duan, J.M.; Liu, S.X.; Li, W.; van Leeuwen, J.; Mulcahy, D. Removal of As(III) and As(V) by ferric salts coagulation—Implications of particle size and zeta potential of precipitates. Sep. Purif. Technol. 2014, 135, 64–71. [Google Scholar] [CrossRef]
- Wang, S.S.; Gao, B.; Zimmerman, A.R.; Li, Y.C.; Ma, L.; Harris, W.G.; Migliaccio, K.W. Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite. Bioresour. Technol. 2015, 175, 391–395. [Google Scholar] [CrossRef]
- Chen, W.F.; Parette, R.; Zou, J.Y.; Cannon, F.S.; Dempsey, B.A. Arsenic removal by iron-modified activated carbon. Water Res. 2007, 41, 1851–1858. [Google Scholar] [CrossRef]
- Ocinski, D.; Jacukowicz-Sobala, I.; Mazur, P.; Raczyk, J.; Kociolek-Balawejder, E. Water treatment residuals containing iron and manganese oxides for arsenic removal from water—Characterization of physicochemical properties and adsorption studies. Chem. Eng. J. 2016, 294, 210–221. [Google Scholar] [CrossRef]
- Li, Z.H.; Jean, J.S.; Jiang, W.T.; Chang, P.H.; Chen, C.J.; Liao, L.B. Removal of arsenic from water using Fe-exchanged natural zeolite. J. Hazard. Mater. 2011, 187, 318–323. [Google Scholar] [CrossRef]
- Yu, W.T.; Luo, M.B.; Yang, Y.X.; Wu, H.; Huang, W.; Zeng, K.; Luo, F. Metal-organic framework (MOF) showing both ultrahigh As(V) and As(III) removal from aqueous solution. J. Solid State Chem. 2019, 269, 264–270. [Google Scholar] [CrossRef]
- Tuchowska, M.; Rzepa, G.; Debiec-Andrzejewska, K.; Drewniak, Ł.; Bajda, T. Immobilization of arsenic compounds by bog iron ores. Desalin. Water Treat. 2019, 157, 138–147. [Google Scholar] [CrossRef] [Green Version]
- Su, X.L.; Ma, L.Y.; Wei, J.M.; Zhu, R.L. Structure and thermal stability of organo-vermiculite. Appl. Clay Sci. 2016, 132, 261–266. [Google Scholar] [CrossRef]
- Li, Z.H.; Bowman, R.S. Retention of inorganic oxyanions by organo-kaolinite. Water Res. 2001, 35, 3771–3776. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, S.J. Adsorption of naphthalene by HDTMA modified kaolinite and halloysite. Appl. Clay Sci. 2002, 22, 55–63. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Z.Y.; Wang, X.; Yan, S.Y.; Wang, J.H.; Fan, Y.W. Preparations of organo-vermiculite with large interlayer space by hot solution and ball milling methods: A comparative study. Appl. Clay Sci. 2011, 51, 151–157. [Google Scholar] [CrossRef]
- Bajda, T.; Klapyta, Z. Adsorption of chromate from aqueous solutions by HDTMA-modified clinoptilolite, glauconite and montmorillonite. Appl. Clay Sci. 2013, 86, 169–173. [Google Scholar] [CrossRef]
- Riebe, B.; Dultz, S.; Bunnenberg, C. Temperature effects on iodine adsorption on orgrano-clay minerals I. Influence of pretreatment and adsorption temperature. Appl. Clay Sci. 2005, 28, 9–16. [Google Scholar] [CrossRef]
- Dultz, S.; An, J.H.; Riebe, B. Organic cation exchanged montmorillonite and vermiculite as adsorbents for Cr (VI): Effect of layer charge on adsorption properties. Appl. Clay Sci. 2012, 67–68, 125–133. [Google Scholar] [CrossRef]
- Liu, S.; Wu, P.X.; Chen, M.Q.; Yu, L.F.; Kang, C.X.; Zhu, N.W.; Dang, Z. Amphoteric modified vermiculites as adsorbents for enhancing removal of organic pollutants: Bisphenol A and Tetrabromobisphenol A. Environ. Pollut. 2017, 228, 277–286. [Google Scholar] [CrossRef]
- Placha, D.; Simha Martynkova, G.; Bachmatiuk, A.; Peikertova, P.; Seidlerova, J.; Rummeli, M.H. The influence of pH on organovermiculite structure stability. Appl. Clay Sci. 2014, 93–94, 17–22. [Google Scholar] [CrossRef]
- Martynkova, G.S.; Valaskova, M.; Capkova, P.; Matejka, V. Structural ordering of organovermiculite: Experiments and modeling. J. Colloid Interface Sci. 2007, 313, 281–287. [Google Scholar] [CrossRef]
- Reeve, P.J.; Fallowfield, H.J. The toxicity of cationic surfactant HDTMA-Br, desorbed from surfactant modified zeolite, towards faecal indicator and environmental microorganisms. J. Hazard. Mater. 2017, 339, 208–215. [Google Scholar] [CrossRef]
- Wolowiec, M.; Muir, B.; Zieba, K.; Bajda, T.; Kowalik, M.; Franus, W. Experimental Study on the Removal of VOCs and PAHs by Zeolites and Surfactant-Modified Zeolites. Energy Fuels 2017, 31, 8803–8812. [Google Scholar] [CrossRef]
- Placha, D.; Martynkova, G.S.; Rummeli, M.H. Preparation of organovermiculites using HDTMA: Structure and sorptive properties using naphthalene. J. Colloid Interface Sci. 2008, 327, 341–347. [Google Scholar] [CrossRef]
- Kooli, F. Thermal stability investigation of organo-acid-activated clays by TG-MS and in situ XRD techniques. Thermochim. Acta 2009, 486, 71–76. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, X.P.; Wang, Q.Q.; Han, Y.J.; Fang, Y.X.; Dong, S.J. Shape-Control of Pt-Ru Nanocrystals: Tuning Surface Structure for Enhanced Electrocatalytic Methanol Oxidation. J. Am. Chem. Soc. 2018, 140, 1142–1147. [Google Scholar] [CrossRef]
- Bergaya, F.; Lagaly, G.; Vayer, M. Cation and anion exchange. In Handbook of Clay Science, 1st ed.; Bergaya, F., Theng, B.K.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 979–1001. [Google Scholar]
- Langmuir, I. The constitution and fundamental properties of solids and liquids Part I Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Uber die Adsorption in Losungen. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Hundakova, M.; Tokarsky, J.; Valaskova, M.; Slobodian, P.; Pazdziora, E.; Kimmer, D. Structure and antibacterial properties of polyethylene/organo-vermiculite composites. Solid State Sci. 2015, 48, 197–204. [Google Scholar] [CrossRef]
- Madejova, J.; Komadel, P. Baseline studies of The Clay Minerals Society Source Clays: Infrared methods. Clays Clay Miner. 2001, 49, 410–432. [Google Scholar] [CrossRef]
- Madejova, J. FTIR techniques in clay mineral studies. Vib. Spectrosc. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- He, H.; Frost, L.R.; Zhu, J. Infrared study of HDTMA+ intercalated montmorillonite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 2853–2859. [Google Scholar]
- Li, Z.H.; Jiang, W.T.; Hong, H.L. An FTIR investigation of hexadecyltrimethylammonium intercalation into rectorite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 71, 1525–1534. [Google Scholar] [CrossRef]
- Shan, Y.; Zeng, P.; Song, Y.; Guo, Y.; Galarneau, A.; Manero, M.-H. Amino-Modified Hydrogen-Bonding Resin and its Adsorption on Berberine. Appl. Mech. Mater. 2013, 448–453, 145–149. [Google Scholar] [CrossRef]
- Aroke, U.O.; El-Nafaty, U. XRF, XRD and FTIR Properties and Characterization of HDTMA-Br Surface Modified Organo-Kaolinite Clay. Int. J. Emerg. Technol. Adv. Eng. 2014, 4, 817–825. [Google Scholar]
- Madejova, J.; Sekerakova, L.; Bizovska, V.; Slany, M.; Jankovic, L. Near-infrared spectroscopy as an effective tool for monitoring the conformation of alkylammonium surfactants in montmorillonite interlayers. Vib. Spectrosc. 2016, 84, 44–52. [Google Scholar] [CrossRef]
- McMurry, J. Organic Chemistry, 7th ed.; Thomson Brooks Cole: Belmont, KY, USA, 2008; Volume 1, p. 1318. [Google Scholar]
- Zhang, H.L.; Yu, J.Y.; Wu, S.P. Effect of montmorillonite organic modification on ultraviolet aging properties of SBS modified bitumen. Constr. Build. Mater. 2012, 27, 553–559. [Google Scholar] [CrossRef]
- Mozgawa, W.; Krol, M.; Bajda, T. IR spectra in the studies of anion sorption on natural sorbents. J. Mol. Struct. 2011, 993, 109–114. [Google Scholar] [CrossRef]
- Wang, J.; Gao, M.; Shen, T.; Yu, M.; Xiang, Y.; Liu, J. Insights into the efficient adsorption of rhodamine B on tunable organo-vermiculites. J. Hazard. Mater. 2019, 15, 501–511. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas Solid Systems with Special Reference to The Determination of Surface-Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Apreutesei, R.E.; Catrinescu, C.; Teodosiu, C. Surfactant-modified natural zeolites for environmental applications in water purification. Environ. Eng. Manag. J. 2008, 7, 149–161. [Google Scholar]
- Perez-Maqueda, L.A.; Balek, V.; Poyato, J.; Perez-Rodriquez, J.L.; Subrt, J.; Bountsewa, I.M.; Beckman, I.N.; Malek, Z. Study of natural and ion exchanged vermiculite by emanation thermal analysis, TG, DTA and XRD. J. Therm. Anal. Calorim. 2003, 71, 715–726. [Google Scholar] [CrossRef]
- Perez-Maqueda, L.A.; Maqueda, C.; Perez-Rodriguez, J.L.; Subrt, J.; Cerny, Z.; Balek, V. Thermal behaviour of ground and unground acid leached vermiculite. J. Therm. Anal. Calorim. 2012, 107, 431–438. [Google Scholar] [CrossRef]
- Liu, S.; Wu, P.X.; Yu, L.F.; Li, L.P.; Gong, B.N.; Zhu, N.W.; Dang, Z.; Yang, C. Preparation and characterization of organo-vermiculite based on phosphatidylcholine and adsorption of two typical antibiotics. Appl. Clay Sci. 2017, 137, 160–167. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, D.; Li, H. Kinetics and thermodynamics of lead(II) adsorption on vermiculite. Sep. Sci. Technol. 2007, 42, 185–202. [Google Scholar] [CrossRef]
- Yu, M.M.; Gao, M.L.; Shen, T.; Wang, J. Organo-vermiculites modified by low-dosage Gemini surfactants with different spacers for adsorption toward p-nitrophenol. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 601–611. [Google Scholar] [CrossRef]
- Wu, N.; Wu, L.M.; Liao, L.B.; Lv, G.C. Organic intercalation of structure modified vermiculite. J. Colloid Interface Sci. 2015, 457, 264–271. [Google Scholar] [CrossRef]
- Lemic, J.; Tomasevic-Canovic, M.; Adamovic, M.; Kovacevic, D.; Milicevic, S. Competitive adsorption of polycyclic aromatic hydrocarbons on organo-zeolites. Microporous Mesoporous Mater. 2007, 105, 317–323. [Google Scholar] [CrossRef]
- Xu, B.W.; Ma, H.Y.; Lu, Z.Y.; Li, Z.J. Paraffin/expanded vermiculite composite phase change material as aggregate for developing lightweight thermal energy storage cement-based composites. Appl. Energy 2015, 160, 358–367. [Google Scholar] [CrossRef]
- Wang, J.; Gao, M.L.; Ding, F.; Shen, T. Organo-vermiculites modified by heating and gemini pyridinium surfactants: Preparation, characterization and sulfamethoxazole adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2018, 546, 143–152. [Google Scholar] [CrossRef]
- Kyzioł, J. Minerały Ilaste Jako Sorbenty Metali Ciężkich; Zakład Narodowy im; Ossolińskich; Wydawnictwo Polskiej Akademii Nauk: Wrocław, Poland, 1994; Volume 43, p. 89. [Google Scholar]
- El-Bayaa, A.A.; Badawy, N.A.; AlKhalik, E.A. Effect of ionic strength on the adsorption of copper and chromium ions by vermiculite pure clay mineral. J. Hazard. Mater. 2009, 170, 1204–1209. [Google Scholar] [CrossRef]
- Shirvani, M.; Kalbasi, M.; Shariatmadari, H.; Nourbakhsh, F.; Najafi, B. Sorption-desorption of cadmium in aqueous palygorskite, sepiolite, and calcite suspensions: Isotherm hysteresis. Chemosphere 2006, 65, 2178–2184. [Google Scholar] [CrossRef]
- Czimerova, A.; Bujdak, J.; Dohrmann, R. Traditional and novel methods for estimating the layer charge of smectites. Appl. Clay Sci. 2006, 34, 2–13. [Google Scholar] [CrossRef]
- Peng, T.J.; Sun, H.J.; Sun, J.M.; Liu, H.F. Chemical Reaction Characteristics of HDTMA+ Cations in Inter layer Space of Vermiculite Crystal Layers. Adv. Mater. Res. 2010, 96, 15–20. [Google Scholar] [CrossRef]
- Jaynes, W.F.; Boyd, S.A. Clay Mineral Type and Organic-Compound Sorption by Hexadecyltrimethlyammonium-Exchanged Clays. Soil Sci. Soc. Am. J. 1991, 55, 43–48. [Google Scholar] [CrossRef]
- Slade, P.G.; Gates, W.P. The swelling of HDTMA smectites as influenced by their preparation and layer charges. Appl. Clay Sci. 2004, 25, 93–101. [Google Scholar] [CrossRef]
- Abate, G.; dos Santos, L.B.O.; Colombo, S.M.; Masini, J.C. Removal of fulvic acid from aqueous media by adsorption onto modified vermiculite. Appl. Clay Sci. 2006, 32, 261–270. [Google Scholar] [CrossRef]
- Dultz, S.; Riebe, B.; Bunnenberg, C. Temperature effects on iodine adsorption on organo-clay minerals II. Structural effects. Appl. Clay Sci. 2005, 28, 17–30. [Google Scholar] [CrossRef]
- Wei, S.Y.; Deng, X.H. Surface charge and adsorption characteristics for fluoride of hexadecyltrimethylammonium modified vermiculite. Adv. Mater. Res. 2013, 781–784, 2265–2268. [Google Scholar] [CrossRef]
- Matusik, J.; Klapyta, Z. Characterization of kaolinite intercalation compounds with benzylalkylammonium chlorides using XRD, TGA/DTA and CHNS elemental analysis. Appl. Clay Sci. 2013, 83–84, 433–440. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, S.J. Delamination behavior of silicate layers by adsorption of cationic surfactants. J. Colloid Interface Sci. 2002, 248, 231–238. [Google Scholar] [CrossRef]
- Hu, Z.W.; He, G.H.; Liu, Y.F.; Dong, C.X.; Wu, X.M.; Zhao, W. Effects of surfactant concentration on alkyl chain arrangements in dry and swollen organic montmorillonite. Appl. Clay Sci. 2013, 75–76, 134–140. [Google Scholar] [CrossRef]
- Gammoudi, S.; Frini-Srasra, N.; Srasra, E. Influence of exchangeable cation of smectite on HDTMA adsorption: Equilibrium, kinetic and thermodynamic studies. Appl. Clay Sci. 2012, 69, 99–107. [Google Scholar] [CrossRef]
- Bartelt-Hunt, S.L.; Burns, S.E.; Smith, J.A. Nonionic organic solute sorption onto two organobentonites as a function of organic-carbon content. J. Colloid Interface Sci. 2003, 266, 251–258. [Google Scholar] [CrossRef]
- Yu, X.B.; Wei, C.H.; Ke, L.; Hu, Y.; Xie, X.Q.; Wu, H.Z. Development of organovermiculite-based adsorbent for removing anionic dye from aqueous solution. J. Hazard. Mater. 2010, 180, 499–507. [Google Scholar] [CrossRef]
- Thanos, A.G.; Sotiropoulos, A.; Malamis, S.; Katsou, E.; Pavlatou, E.A.; Haralambous, K.J. Regeneration of HDTMA-modified minerals after sorption with chromate anions. Desalin. Water Treat. 2016, 57, 27869–27878. [Google Scholar] [CrossRef]
- Manning, B.A.; Goldberg, S. Modeling arsenate competitive adsorption on kaolinite, montmorillonite and illite. Clays Clay Miner. 1996, 44, 609–623. [Google Scholar] [CrossRef]
- Abollino, O.; Giacomino, A.; Malandrino, M.; Mentasti, E. Interaction of metal ions with montmorillonite and vermiculite. Appl. Clay Sci. 2008, 38, 227–236. [Google Scholar] [CrossRef]
- Dos Anjos, V.E.; Rohwedder, J.R.; Cadore, S.; Abate, G.; Grassi, M.T. Montmorillonite and vermiculite as solid phases for the preconcentration of trace elements in natural waters: Adsorption and desorption studies of As, Ba, Cu, Cd, Co, Cr, Mn, Ni, Pb, Sr, V, and Zn. Appl. Clay Sci. 2014, 99, 289–296. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef] [Green Version]
- Ben Issa, N.; Rajakovic-Ognjanovic, V.N.; Jovanovic, B.M.; Rajakovic, L.V. Determination of inorganic arsenic species in natural waters-Benefits of separation and preconcentration on ion exchange and hybrid resins. Anal. Chim. Acta 2010, 673, 185–193. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Li, J.X.; Sun, C.Z.; Marhaba, T.F.; Zhang, W.; Zhang, Y.H. Arsenic Speciation by Sequential Extraction from As-Fe Precipitates Formed Under Different Coagulation Conditions. Water Air Soil Pollut. 2016, 227, 9. [Google Scholar] [CrossRef]
- Yusof, A.M.; Malek, N. Removal of Cr(VI) and As(V) from aqueous solutions by HDTMA-modified zeolite Y. J. Hazard. Mater. 2009, 162, 1019–1024. [Google Scholar] [CrossRef]
- Sposito, G.; Skipper, N.T.; Sutton, R.; Park, S.H.; Soper, A.K.; Greathouse, J.A. Surface geochemistry of the clay minerals. Proc. Natl. Acad. Sci. USA 1999, 96, 3358–3364. [Google Scholar] [CrossRef] [Green Version]
- Wainipee, W.; Cuadros, J.; Sephton, M.A.; Unsworth, C.; Gill, M.G.; Strekopytov, S.; Weiss, D.J. The effects of oil on As (V) adsorption on illite, kaolinite, montmorillonite and chlorite. Geochim. Cosmochim. Acta 2013, 121, 487–502. [Google Scholar] [CrossRef]
- Mohapatra, D.; Mishra, D.; Chaudhury, G.R.; Das, R.P. Arsenic adsorption mechanism on clay minerals and its dependence on temperature. Korean J. Chem. Eng. 2007, 24, 426–430. [Google Scholar] [CrossRef]
- Greenleaf, J.E.; Cumbal, L.; Staina, I.; SenGupta, A.K. Abiotic As(III) oxidation by hydrated Fe(III) oxide (HFO) microparticles in a plug flow columnar configuration. Process Saf. Environ. Prot. 2003, 81, 87–98. [Google Scholar] [CrossRef]
- Raczyński, W.W. Zarys Teorii Dynamiki Sorpcji i Chromatografii, 1st ed.; Wydawnictwo Naukowo-Techniczne Warszawa: Warszawa, Poland, 1966. [Google Scholar]
- Maślankiewicz, K.; Szymański, A. Mineralogia Stosowana; Wydawnictwo Geologiczne: Warszawa, Poland, 1976; p. 464. [Google Scholar]











| Parameter | HDTMA | HDBA | ||||
|---|---|---|---|---|---|---|
| Multiple of CEC | 0.5 | 1.0 | 2.0 | 0.5 | 1.0 | 2.0 |
| Amount of organic salt (g) | 4.9 | 9.7 | 19.5 | 5.3 | 10.6 | 21.2 |
| Sample | SBET (m2/g) | Dp (nm) | Vmes (cm3/g) |
|---|---|---|---|
| VER | 7.34 | 10.48 | 0.0138 |
| 0.5 HDTMA | 3.49 | 14.35 | 0.0070 |
| 2.0 HDTMA | 4.23 | 11.35 | 0.0026 |
| 0.5 HDBA | 15.47 | 5.55 | 0.0175 |
| 2.0 HDBA | 5.93 | 4.66 | 0.0053 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuchowska, M.; Wołowiec, M.; Solińska, A.; Kościelniak, A.; Bajda, T. Organo-Modified Vermiculite: Preparation, Characterization, and Sorption of Arsenic Compounds. Minerals 2019, 9, 483. https://doi.org/10.3390/min9080483
Tuchowska M, Wołowiec M, Solińska A, Kościelniak A, Bajda T. Organo-Modified Vermiculite: Preparation, Characterization, and Sorption of Arsenic Compounds. Minerals. 2019; 9(8):483. https://doi.org/10.3390/min9080483
Chicago/Turabian StyleTuchowska, Magdalena, Magdalena Wołowiec, Agnieszka Solińska, Anita Kościelniak, and Tomasz Bajda. 2019. "Organo-Modified Vermiculite: Preparation, Characterization, and Sorption of Arsenic Compounds" Minerals 9, no. 8: 483. https://doi.org/10.3390/min9080483
APA StyleTuchowska, M., Wołowiec, M., Solińska, A., Kościelniak, A., & Bajda, T. (2019). Organo-Modified Vermiculite: Preparation, Characterization, and Sorption of Arsenic Compounds. Minerals, 9(8), 483. https://doi.org/10.3390/min9080483