Mustard Gold of the Gaching Ore Deposit (Maletoyvayam Ore Field, Kamchatka, Russia)
Abstract
:1. Introduction
2. Materials and Methods
3. Results
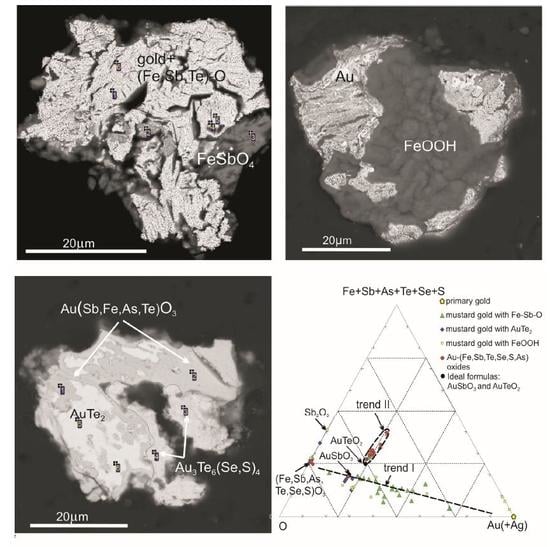
3.1. Types of Mustard Gold
3.2. Transformation Sequence from Calaverite to Gold
4. Discussion
- (1)
- AuTe2 + Fe, Sb, Bi, As, Se, and S-containing solutions + O2 → Au + Te,Se solid solution + TeO2 + Fe(Sb,As)O3 as a composite of a gold sponge and Sb–Fe oxide ± admixtures.
- (2)
- AuTe2 + Fe, Sb, Bi, As, Se, and S-containing solutions + O2 → Au(Te,Fe,Se,Sb,S)O2.
5. Conclusions
- (a)
- Mustard gold with inclusions of the oxides of Sb, Te(Se,S), and Fe (Fe-antimonate/tellurate) infilling the pores of spongy gold—the early (hypogene) transformation stage of calaverite due to the impact of Fe, Sb, Te, As, Se, and S-containing hydrothermal solutions and high oxidation potential.
- (b)
- Spotted and colloform gold consisting of aggregates of small particles of gold in a goethite/hydrogoethite matrix—the late (possibly hypergene) transformation stage associated with the maximum degree of ore oxidation.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lindgren, W. Mineral Deposits; McGraw-Hill Book Company, Inc.: New York, NY, USA; London, UK, 1933; p. 930. [Google Scholar]
- Sotnikov, V.I. Gold in the bedrock source—Placer system. Soros Educ. J. 1998, 5, 66–71. (In Russian) [Google Scholar]
- Nekrasov, I.Y. Geochemistry, Mineralogy and Genesis of Gold Deposits; Nauka: Moscow, Russia, 1991; p. 302. (In Russian) [Google Scholar]
- Okrugin, V.M.; Andreeva, E.D.; Yablokova, D.A.; Okrugina, A.M.; Chubarov, V.M.; Ananiev, V.V. The new data on the ores of the Aginskoye gold-telluride deposit (Central Kamchatka). In “Volcanism and Its Associated Processes” Conference; Petropavlovsk-Kamchatsky: Kamchatka Krai, Russia, 2014; pp. 335–341. (In Russian) [Google Scholar]
- Okrugin, V.M.; Andreeva, E.; Etschmann, B.; Pring, A.; Li, K.; Zhao, J.; Griffiths, G.; Lumpkin, G.R.; Triani, G.; Brugger, J. Microporous gold: Comparison of textures from Nature and experiments. Am. Mineral. 2014, 99, 1171–1174. [Google Scholar] [CrossRef]
- Kudaeva, A.L.; Andreeva, E.D. Mustard gold: Characteristics, types and chemical composition. In The Natural Environment of Kamchatka; Petropavlovsk-Kamchatsky: Kamchatka Krai, Russia, 2014; pp. 17–29. (In Russian) [Google Scholar]
- Wilson, A.F. Origin of quartz-free gold nuggets and supergene gold found in laterites and soils—A review and some new observations. Aust. J. Earth Sci. 1984, 31, 303–316. [Google Scholar]
- Litvinenko, I.S.; Shilina, L.A. Hypergene gold neomineralization in placer deposits of Nizhne-Myakitsky ore-placer field, North-East Russia. J. Ores Met. 2017, 1, 75–90. (In Russian) [Google Scholar]
- Petersen, S.O.; Makovicky, E.; Juiling, L.; Rose-Hansen, J. Mustard gold from the Dongping Au-Te deposit, Hebei province, People’s Republic of China. N. Jb. Mineral. Mh. 1999, 8, 337–357. [Google Scholar]
- Li, J.; Makovicky, E. New studies on mustard gold from the Dongping Mines, Hebei Province, China: The tellurian, plumbian, manganoan and mixed varieties. N. Jb. Mineral. Abh. 2001, 176, 269–297. [Google Scholar]
- Gamyanin, G.N.; Zhdanov, Y.Y.; Nekrasov, T.Y.; Leskova, N.V. “Mustard” gold from gold-antimony ores of Eastern Yakutia. New Data Miner. 1987, 34, 13–20. (In Russian) [Google Scholar]
- Dill, H.G.; Weiser, T.; Benhardt, I.R.; Kilibarda, R. The composite gold—Antimony vein deposit at Kharma (Bolivia). Econ. Geol. 1995, 90, 51–66. [Google Scholar] [CrossRef]
- Wang, D.; Liu, J.; Zhai, D.; Carranza, E.J.M.; Wang, Y.; Zhen, S.; Wang, J.; Wang, J.; Liu, Z.; Zhang, F. Mineral Paragenesis and Ore-Forming Processes of the Dongping Gold Deposit, Hebei Province, China. Resour. Geol. 2019, 29, 287–313. [Google Scholar] [CrossRef]
- Tolstykh, N.; Vymazalova, A.; Tuhy, M.; Shapovalova, M. Conditions of formation of Au–Se–Te mineralization in the Gaching ore occurrence (Maletoyvayam ore field), Kamchatka, Russia. Mineral. Mag. 2018, 82, 649–674. [Google Scholar] [CrossRef]
- Tsukanov, N.V. Tectono-stratigraphic terranes of Kamchatka active margins: Structure, composition and geodynamics. In Materials of the Annual Conference “Volcanism and Related Processes”; Petropavlovsk-Kamchatsky: Kamchatka Krai, Russia, 2015; pp. 97–103. (In Russian) [Google Scholar]
- Golyakov, V.I. Geological Map of the USSR Scale 1: 200,000; Pogozhev, A.G., Ed.; Series Koryak; Sheets P-5 8-XXXIII, O-58-III; VSEGEI Cartographic Factory: St. Petersburg, Russia, 1980. (In Russian) [Google Scholar]
- Melkomukov, B.H.; Razumny, A.V.; Litvinov, A.P.; Lopatin, W.B. New highly promising gold objects of Koryakiya. Min. Bull. Kamchatka 2010, 14, 70–74. (In Russian) [Google Scholar]
- Palyanova, G.A.; Tolstykh, N.D.; Zinina, V.Y.; Kokh, K.A.; Seryotkin, Y.V.; Bortnikov, N.S. Synthetic chalcogenides of gold in the Au-Te-Se-S system and their natural analogs. Dokl. Earth Sci. 2019, 487, 929–934. [Google Scholar]
- Tolstykh, N.D.; Tuhý, M.; Vymazalová, A.; Plášil, J.; Laufek, F.; Kasatkin, A.V.; Nestola, F. Maletoyvayamite, IMA 2019-021. CNMNC Newsletter No. 50. Mineral. Mag. 2019, 31. [Google Scholar] [CrossRef]
- Kovalenker, V.A.; Nekrasov, I.Y.; Sandomirskaya, S.M.; Nekrasova, A.N.; Malov, V.S.; Danchenko, V.Y.; Dmitrieva, M.T. Sulfide-selenide-telluride mineralization of epithermal ores in the Kuril-Kamchatka volcanic belt. Mineral. J. 1989, 11, 3–18. (In Russian) [Google Scholar]
- Basso, R.; Cabella, R.; Lucchetti, G.; Marescotti, P.; Martinelli, A. Structural studies on synthetic and natural Fe–Sb–oxides of MO2 type. N. Jb. Mineral. Mh. 2003, 407–420. [Google Scholar] [CrossRef]
- Kalinin, Y.A.; Palyanova, G.A.; Naumov, E.A.; Kovalev, K.R.; Pirajno, F. Supergene remobilization of Au in Au-bearing regolith related to orogenic deposits: A case study from Kazakhstan. Ore Geol. Rev. 2019, 109, 358–369. [Google Scholar] [CrossRef]
- Cabri, L.J. Phase relations in the Au-Ag-Te system and their mineralogical significance. Econ. Geol. 1965, 60, 1569–1605. [Google Scholar] [CrossRef]
- Makovicky, E.; Chovan, M.; Bakos, F. The stibian mustard gold from the Krivánˇ Au deposit, Tatry Mts., Slovak Republic. N. Jb. Mineral. Abh. 2007, 184, 207–215. [Google Scholar]
- Gamyanin, G.N.; Nekrasov, I.; Zhdanov, J.J.; Leskova, I.V. Auroantimonate—A new natural compound of gold. Dokl. Akad Nauk SSSR Mineral. 1988, 301, 947–950. (In Russian) [Google Scholar]
- Tian, S.; Li, X. A preliminary study of some new minerals in Dongping gold deposit, Hebei province. Gold Geol. 1995, 1, 61–67. [Google Scholar]
- Johan, Z.; Šrein, V. Un nouvel oxide naturel de Au et Sb. Sci. Terre Planetes 1998, 326, 533–538. [Google Scholar]
- Zacharias, J.; Mate, J.N. Gold to aurostibite transformation and formation of Au-Ag-Sb phases: The Krásná Hora deposit, Czech Republic. Mineral. Mag. 2017, 81, 987–999. [Google Scholar] [CrossRef]
- Zhao, J.; Brugger, J.; Pascal, V.; Gundler, P.; Xia, F.; Chen, G.; Pring, A. Mechanism and kinetics of a mineral transformation under hydrothermal conditions: Calaverite to metallic gold. Am. Mineral. 2009, 94, 1541–1555. [Google Scholar] [CrossRef]
- Zhao, J.; Xia, F.; Pring, A.; Brugger, J.; Grundler, P.V.; Chen, G. A novel pretreatment of calaverite by hydrothermal mineral replacement reactions. Miner. Eng. 2010, 23, 451–453. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, J.; Brugger, J.; Chen, G.; Pring, A. Mechanism of mineral transformations in krennerite, Au3AgTe8, under hydrothermal conditions. Am. Mineral. 2013, 98, 2086–2095. [Google Scholar] [CrossRef]
- Xia, F.; Brugger, J.; Chen, G.; Ngothai, Y.; O’Neill, B.; Putnis, A.; Pring, A. Mechanism and kinetics of pseudomorphic mineral replacement reactions: A case study of the replacement of pentlandite by violarite. Geochim. Cosmochim. Acta 2009, 73, 1945–1969. [Google Scholar] [CrossRef]
- Zhao, J.; Pring, A. Mineral Transformations in Gold–(Silver) Tellurides in the Presence of Fluids: Nature and Experiment. Minerals 2019, 9, 167. [Google Scholar] [CrossRef]
- Сooke, D.R.; McPhail, D.C. Epithermal Au–Ag–Te mineralization, Acupan, Baguio District, Philippines; numerical simulations of mineral deposition. Econ. Geol. 2001, 96, 109–131. [Google Scholar]
- Kongolo, K.; Mwema, M.D. The extractive metallurgy of gold. Hyperfine Interact. 1998, 111, 281–289. [Google Scholar] [CrossRef]
- Grosse, A.C.; Dicinoski, G.W.; Shaw, M.J.; Haddad, P.R. Leaching and recovery of gold using ammoniacal thiosulfate leach liquors (a review). Hydrometallurgy 2003, 69, 1–21. [Google Scholar] [CrossRef]










| No. | Sample | Sp. | Cu | Au | Ag | Bi | Sb | Te | As | Se | S | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3_3 | 1 | - | 98.45 | 1.86 | - | - | - | - | - | - | 100.31 |
| 2 | 3_3 | 2 | - | 96.23 | 1.98 | - | - | - | - | - | - | 98.21 |
| 3 | 3_3 | 3 | 41.81 | - | 0.37 | - | 19.4 | - | 6.51 | 4.64 | 27.76 | 100.49 |
| 4 | 3_3 | 4 | - | 36.25 | - | - | - | 49.35 | - | 5.05 | 8.6 | 99.25 |
| 5 | 4_1 | 1 | - | 98.54 | 1.23 | - | - | - | - | - | - | 99.77 |
| 6 | 4_1 | 2 | - | 100.33 | 1.61 | - | - | - | - | - | - | 101.94 |
| 7 | 4_1 | 3 | - | 35.92 | - | - | - | 47.67 | - | 15.35 | 2.05 | 100.99 |
| 8 | 4_1 | 4 | - | 36.61 | - | - | - | 48.48 | - | 11.16 | 4.29 | 100.54 |
| 9 | 13_1 | 1 | - | 98.1 | 1.88 | - | - | - | - | - | - | 99.98 |
| 10 | 13_1 | 2 | 1.06 | 34.8 | - | 1.28 | - | 47.35 | - | 12.24 | 3.38 | 100.11 |
| 11 | 13_1 | 3 | - | 35.41 | - | - | - | 45.48 | - | 15.77 | 1.03 | 97.69 |
| 12 | 13_1 | 4 | - | 96.05 | 1.55 | - | - | - | - | - | - | 97.6 |
| 13 | 13_1 | 5 | - | 35.45 | - | - | - | 45.64 | - | 14.61 | 1.71 | 97.41 |
| 14 | 13_1 | 6 | - | 37.41 | - | 0.92 | - | 46.47 | - | 9.91 | 3.94 | 98.65 |
| 15 | 13_1 | 7 | 0.19 | 35.58 | - | 0.75 | - | 46.39 | - | 13.28 | 2.65 | 98.84 |
| 16 | 13_1 | 8 | 0.25 | 35.26 | - | - | - | 45.67 | - | 14.66 | 1.77 | 97.61 |
| 17 | 13_1 | 9 | 41.89 | - | - | - | 19.29 | - | 4.72 | 4.36 | 26.97 | 98.38 |
| 18 | 11_3 | 1 | - | 98.52 | 1.52 | - | - | - | - | - | - | 100.04 |
| 19 | 11_3 | 2 | - | 97.6 | 1.47 | - | - | - | - | - | - | 99.07 |
| 20 | 11_3 | 4 | - | 35.09 | - | 0.74 | - | 45.96 | - | 14.56 | 1.32 | 97.67 |
| 21 | 11_3 | 5 | - | 69.91 | - | - | - | 1.09 | - | 26.04 | 2.37 | 99.41 |
| 22 | 11_3 | 6 | - | 35.93 | - | - | - | 47.78 | - | 13.6 | 3.32 | 100.63 |
| 23 | 11_3 | 7 | - | 35.53 | - | 0.81 | - | 47.12 | - | 13.23 | 2.76 | 99.45 |
| 24 | 11_3 | 9 | - | 37.31 | - | 0.74 | - | 46.6 | - | 10.99 | 3.84 | 99.48 |
| 25 | 11_3 | 10 | - | 66.71 | - | - | - | 18.2 | - | 14.78 | - | 99.69 |
| No. | Formula | Abbr. | No. | Formula | Abbr. |
|---|---|---|---|---|---|
| 1 | Au0.97Ag0.03 | gd | 14 | Au3.06Te5.87(Se2.02S1.98 Bi0.07)4.07 | Mt |
| 2 | Au0.97Ag0.03 | gd | 15 | (Au2.93Cu0.05)2.98Te5.90(Se2.73S1.34 Bi0.06)4.13 | Mt |
| 3 | Cu3.95Sb0.96(As0.52Se0.35)0.87S5.20 | wt | 16 | (Au2.98Cu0.07)3.05Te5.95(Se3.09S0.92)4.01 | Mt |
| 4 | Au1.02Te2.14(S1.48Se0.35)1.87 | unn | 17 | Cu4.08Sb0.98(As0.39Se0.34)0.73S5.21 | Wt |
| 5 | Au0.98Ag0.02 | gd | 18 | Au0.97Ag0.03 | Gd |
| 6 | Au0.97Ag0.03 | gd | 19 | Au0.97Ag0.03 | Gd |
| 7 | Au2.91Te5.95(Se3.10S1.02)4.12 | mt | 20 | Au3.02Te6.10(Se3.12S0.70 Bi0.06)3.88 | Mt |
| 8 | Au1.99Te4.07(Se1.51S1.43)2.94 | unn | 21 | Au0.93(Se0.86S0.19Te0.02)1.07 | Unn |
| 9 | Au0.97Ag0.03 | gd | 22 | Au1.97Te4.05(Se1.86S1.12)2.98 | Unn |
| 10 | (Au2.76Cu0.26)3.02Te5.81(Se2.43S1.65 Bi0.10)4.18 | mt | 23 | Au2.91Te5.95(Se2.70S1.39 Bi0.06)4.15 | Mt |
| 11 | Au3.04Te6.03(Se3.38S0.54)3.92 | mt | 24 | Au3.01Te5.81(Se2.21S1.91 Bi0.06)4.18 | Mt |
| 12 | Au0.97Ag0.03 | gd | 25 | Au2.03Te0.85Se1.12 | Unn |
| 13 | Au3.02Te5.99(Se3.10S0.89)3.99 | - | - | - | - |
| Sample | Sp. | Fe | Au | Ag | Bi | Sb | Te | As | S | O | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3_11 | 2 | 5.93 | 76.70 | 2.33 | - | 2.23 | 2.32 | 1.40 | 0.59 | 7.13 | 98.63 |
| 3_11 | 3 | 2.18 | 88.66 | 0.70 | - | 1.00 | 1.28 | 0.80 | - | 5.61 | 100.23 |
| 3_11 | 4 | 6.81 | 78.07 | 0.84 | - | 1.13 | 1.72 | 0.79 | - | 9.03 | 98.39 |
| 3_11 | 5 | 4.76 | 79.85 | 0.78 | - | 1.69 | 2.06 | 1.46 | - | 9.62 | 100.22 |
| 3_12 | 2 | 4.45 | 82.36 | 0.72 | - | 2.12 | 1.97 | 1.05 | - | 6.74 | 99.41 |
| 3_12 | 4 | 4.62 | 81.42 | 1.00 | - | 2.16 | 2.12 | 0.80 | - | 6.05 | 98.17 |
| 8_7 | 1 | 4.58 | 70.20 | 1.40 | 2.60 | 9.02 | 1.00 | 1.43 | 0.30 | 9.42 | 99.95 |
| 8_7 | 2 | 5.17 | 69.63 | 1.47 | 2.53 | 9.58 | 0.70 | 1.72 | 0.39 | 9.53 | 100.72 |
| 8_7 | 3 | 6.63 | 62.91 | 1.46 | 2.02 | 11.42 | 1.00 | 2.28 | 0.41 | 12.70 | 100.83 |
| 8_7 | 4 | 5.73 | 67.88 | 1.32 | 2.58 | 10.26 | 1.21 | 2.01 | 0.34 | 9.57 | 100.90 |
| 8_7 | 5 | 6.62 | 63.82 | 1.32 | 2.07 | 11.11 | 1.10 | 2.15 | 0.35 | 10.58 | 99.12 |
| 8_7 | 6 | 5.44 | 63.65 | 1.58 | 2.72 | 11.41 | 1.22 | 2.06 | - | 11.85 | 99.93 |
| 9_6 | 1 | 5.32 | 86.15 | - | - | - | - | 0.63 | - | 6.66 | 98.76 |
| 9_6 | 2 | 15.83 | 41.44 | - | 1.53 | 14.45 | 1.44 | 2.06 | 0.33 | 23.23 | 100.31 |
| 9_6 | 4 | 4.34 | 85.16 | - | - | - | - | - | - | 8.78 | 98.28 |
| 9_6 | 5 | 1.58 | 96.52 | - | - | - | - | - | - | 2.88 | 100.98 |
| 9_6 | 6 | 4.39 | 85.92 | - | - | 0.70 | - | 0.61 | - | 8.80 | 100.42 |
| Sample | Sp. | Fe | Au | Ag | Bi | Sb | Te | As | S | O | Total |
| at. % | |||||||||||
| 3_11 | 2 | 9.87 | 38.18 | 2.12 | - | 1.80 | 1.78 | 1.83 | 0.73 | 43.69 | 100 |
| 3_11 | 3 | 4.24 | 51.55 | 0.74 | - | 0.94 | 1.15 | 1.22 | - | 40.16 | 100 |
| 3_11 | 4 | 10.34 | 35.47 | 0.70 | - | 0.83 | 1.21 | 0.94 | - | 50.51 | 100 |
| 3_11 | 5 | 7.06 | 35.43 | 0.63 | - | 1.21 | 1.41 | 1.70 | - | 52.55 | 100 |
| 3_12 | 2 | 7.80 | 43.18 | 0.69 | - | 1.80 | 1.59 | 1.45 | - | 43.50 | 100 |
| 3_12 | 4 | 8.48 | 44.73 | 1.00 | - | 1.92 | 1.80 | 1.16 | - | 40.91 | 100 |
| 8_7 | 1 | 6.71 | 30.76 | 1.12 | 1.07 | 6.39 | 0.68 | 1.65 | 0.81 | 50.81 | 100 |
| 8_7 | 2 | 7.42 | 29.91 | 1.15 | 1.02 | 6.66 | 0.46 | 1.94 | 1.03 | 50.40 | 100 |
| 8_7 | 3 | 8.07 | 22.92 | 0.97 | 0.69 | 6.73 | 0.56 | 2.18 | 0.92 | 56.95 | 100 |
| 8_7 | 4 | 8.13 | 28.82 | 1.02 | 1.03 | 7.05 | 0.79 | 2.24 | 0.89 | 50.02 | 100 |
| 8_7 | 5 | 8.92 | 25.73 | 0.97 | 0.79 | 7.25 | 0.68 | 2.28 | 0.87 | 52.51 | 100 |
| 8_7 | 6 | 7.02 | 24.58 | 1.11 | 0.99 | 7.13 | 0.73 | 2.09 | - | 56.34 | 100 |
| 9_6 | 1 | 9.48 | 45.93 | - | - | 0.00 | 0.00 | 0.88 | - | 43.71 | 100 |
| 9_6 | 2 | 12.76 | 9.99 | - | 0.35 | 5.64 | 0.54 | 1.31 | 0.49 | 68.94 | 100 |
| 9_6 | 4 | 6.98 | 40.99 | - | - | - | - | - | - | 52.03 | 100 |
| 9_6 | 5 | 3.85 | 70.32 | - | - | - | - | - | - | 25.83 | 100 |
| 9_6 | 6 | 6.93 | 40.59 | - | - | 0.54 | - | 0.76 | - | 51.18 | 100 |
| Sample | Sp. | Fe | Bi | Sb | Te | As | S | O | Total |
|---|---|---|---|---|---|---|---|---|---|
| 3_11 | 2 | 16.53 | - | 3.02 | 2.98 | 3.07 | 1.22 | 73.18 | 100 |
| 3_11 | 3 | 8.89 | - | 1.97 | 2.41 | 2.56 | - | 84.18 | 100 |
| 3_11 | 4 | 16.20 | - | 1.30 | 1.90 | 1.47 | - | 79.13 | 100 |
| 3_11 | 5 | 11.04 | - | 1.89 | 2.21 | 2.66 | - | 82.20 | 100 |
| 3_12 | 2 | 13.89 | - | 3.21 | 2.83 | 2.58 | - | 77.48 | 100 |
| 3_12 | 4 | 15.63 | - | 3.54 | 3.32 | 2.14 | - | 75.38 | 100 |
| 8_7 | 1 | 9.85 | 1.57 | 9.38 | 1.00 | 2.42 | 1.19 | 74.59 | 100 |
| 8_7 | 2 | 10.76 | 1.48 | 9.66 | 0.67 | 2.81 | 1.49 | 73.12 | 100 |
| 8_7 | 3 | 10.60 | 0.91 | 8.84 | 0.74 | 2.86 | 1.21 | 74.84 | 100 |
| 8_7 | 4 | 11.59 | 1.47 | 10.05 | 1.13 | 3.19 | 1.27 | 71.30 | 100 |
| 8_7 | 5 | 12.17 | 1.08 | 9.89 | 0.93 | 3.11 | 1.19 | 71.64 | 100 |
| 8_7 | 6 | 9.45 | 1.33 | 9.60 | 0.98 | 2.81 | - | 75.83 | 100 |
| 9_6 | 1 | 17.53 | - | - | - | 1.63 | - | 80.84 | 100 |
| 9_6 | 2 | 14.17 | 0.39 | 6.26 | 0.60 | 1.46 | 0.54 | 76.57 | 100 |
| 9_6 | 4 | 11.83 | - | - | - | - | - | 88.17 | 100 |
| 9_6 | 5 | 12.97 | - | - | - | - | - | 87.03 | 100 |
| 9_6 | 6 | 11.66 | - | 0.91 | - | 1.28 | - | 86.15 | 100 |
| No. | Sample | Sp. | Fe | Au | O | Total |
|---|---|---|---|---|---|---|
| 1 | 3_1 | 1 | - | 97.18 | - | 97.18 |
| 2 | 3_1 | 2 | - | 97.62 | - | 97.62 |
| 3 | 3_1 | 3 | 52.57 | - | 45.39 | 97.96 |
| 4 | 4_1 | 1 | - | 98.62 | - | 98.62 |
| 5 | 4_1 | 2 | 13.26 | 77.8 | 9.65 | 100.71 |
| 6 | 4_1 | 3 | 51.19 | 0.92 | 45.18 | 97.29 |
| 7 | 4_2 | 1 | 4.95 | 90.18 | 3.99 | 99.12 |
| 8 | 4_5 | 11 | - | 97.57 | - | 97.57 |
| 9 | 4_5 | 2 | 14.11 | 70.06 | 17.48 | 101.65 |
| 10 | 4-5a | 1 | 12.87 | 72.42 | 15.28 | 100.57 |
| 11 | 4-5a | 2 | 12.18 | 72.23 | 16.12 | 100.53 |
| 12 | 4-5a | 3 | 13.14 | 71.36 | 16.06 | 100.56 |
| Sample | Sp. | Fe | Au | Ag | Sb | Te | As | Se | S | O | Total | Abbr. | Formula |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2_3 | 1 | - | 35.25 | - | - | 45.61 | - | 16.63 | 0.34 | - | 97.83 | mt | Au3.07Te6.13(Se3.61S0.18)3.79 |
| 2_3 | 2 | - | 35.32 | - | - | 46.06 | - | 16.32 | 1.13 | - | 98.83 | mt | Au2.98Te6.00(Se3.44S0.59)4.03 |
| 2_3 | 3 | - | 43.52 | 0.41 | - | 52.91 | - | 1.52 | - | - | 98.36 | calv | (Au1.01Ag0.02)1.03(Te1.89Se0.09)1.98 |
| 2_3 | 4 | - | 44.79 | 0.37 | - | 53.38 | - | 1.67 | - | - | 100.21 | calv | (Au1.02Ag0.02)1.04(Te1.87Se0.09)1.96 |
| 2_3 | 5 | - | 43.88 | - | - | 51.97 | - | 1.4 | 0.27 | - | 97.52 | calv | Au1.02(Te1.86Se0.08S0.04)1.98 |
| 2_3 | 6 | 6.62 | 59.19 | 0.96 | 10.1 | 3.53 | 2.43 | - | - | 15.71 | 98.54 | Auox | (Au0.97Ag0.03)1.00 (Fe0.36Sb0.27Te0.10As0.10)0.83O3.17 |
| 7_5 | 2 | - | 44.7 | - | - | 53.45 | - | 1.77 | 0.68 | - | 100.6 | calv | Au0.99(Te1.82Se0.10S0.09)2.01 |
| 8_6 | 1 | - | 42.55 | 0.97 | - | 54.36 | - | 1.87 | 0.22 | - | 99.97 | calv | (Au0.95Ag0.04)0.99(Te1.88Se0.10S0.03)2.01 |
| 8_6 | 2 | - | 41.26 | 0.82 | - | 54.5 | - | 1.93 | 0.27 | - | 98.78 | calv | (Au0.93Ag0.03)0.96(Te1.89Se0.11S0.04)2.04 |
| 8_6 | 3 | - | 36.46 | - | - | 46.71 | - | 14.44 | 1.99 | - | 99.6 | mt | Au3.02Te5.98(Se2.99S1.01)4.00 |
| 8_6 | 4 | - | 37.39 | - | - | 46.89 | - | 10.37 | 3.83 | - | 98.48 | mt | Au3.05Te5.91(Se2.11S1.92)4.03 |
| 8_6 | 5 | 2.64 | 53.84 | - | 2.36 | 29.31 | 0.73 | 0.46 | - | 8.96 | 98.30 | Auox | Au0.96(Te0.80Fe0.16Sb0.07As0.03Se0.02)1.08O1.96 |
| 8_6 | 6 | 1.98 | 58.09 | 0.85 | 1.41 | 30.09 | 0.43 | 0.64 | - | 6.51 | 100.00 | Auox | (Au2.94Ag0.08)3.02(Te2.35Fe0.33Sb0.12Se0.08As0.06)2.94O4.05 |
| 9_11 | 1 | - | 42.58 | - | - | 54.27 | - | 1.61 | - | - | 98.46 | calv | Au0.98(Te1.93Se0.09)2.02 |
| 9_11 | 2 | - | 35.92 | - | - | 46.06 | - | 18.08 | - | - | 100.06 | mt | Au3.07Te6.08Se3.85 |
| 9_11 | 3 | - | 35.24 | - | - | 45.54 | - | 17.6 | - | - | 98.38 | mt | Au3.07Te6.12Se3.82 |
| 9_11 | 4 | - | 42.98 | - | - | 54.27 | - | 1.68 | - | - | 98.93 | calv | Au0.98(Te1.92Se0.10)2.02 |
| 9_11 | 5 | 0.42 | 42.15 | - | - | 53.94 | - | 1.64 | - | - | 98.15 | calv | Au0.97(Te1.91Se0.09Fe0.03)2.03 |
| Sample | Sp. | Fe | Au | Ag | Bi | Sb | Te | As | Se | S | O | Total | Abbr. | Formula |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8_10 | 1 | 4.36 | 56.34 | 2.3 | - | 5.72 | 11.92 | 1.58 | 5.06 | 1.74 | 10.53 | 99.55 | Auox | (Au0.87Ag0.06)0.93(Te0.28Fe0.22Se0.19S0.16Sb0.14As0.06)0.99O2.00 |
| 8_10 | 2 | 2.91 | 55.94 | 2.62 | 1.58 | 6.11 | 13.67 | - | 5.36 | 2.03 | 9.68 | 99.9 | Auox | (Au0.90Ag0.08)0.98(Te0.34Se0.22S0.20Fe0.16Sb0.16Bi0.02)1.10O1.92 |
| 8_10 | 3 | 3.92 | 59.55 | 2.24 | 0.92 | 4.61 | 8.19 | 0.94 | 3.03 | 1.3 | 9.23 | 93.93 | Auox | (Au1.04Ag0.07)1.11(Fe0.23Te0.22S0.14Sb0.13As0.04Bi0.02)0.91O1.98 |
| 8_10 | 4 | 2.15 | 74.83 | 1.67 | 1.77 | 5.34 | 1.31 | - | - | 1.78 | 10.11 | 98.96 | Auox | (Au1.29Ag0.05)1.34(S0.19Sb0.15Fe0.12Te0.03Bi0.03)0.52O2.14 |
| 8_10 | 5 | 2.71 | 54.62 | 2.97 | 1.5 | 3.59 | 17.25 | - | 6.96 | 1.91 | 7.51 | 99.02 | Auox | (Au0.97Ag0.10)1.07(Te0.47Se0.31S0.21Fe0.16Sb0.10Bi0.03)1.28O1.65 |
| 8_10 | 6 | 2.17 | 78.16 | 1.02 | 1.68 | 4.56 | 1.10 | - | - | 1.47 | 7.6 | 97.76 | Auox | (Au1.56Ag0.04)1.60(S0.18Sb0.15Fe0.14Te0.03Bi0.03)0.53O1.87 |
| 8_10 | 9 | 6.26 | 2.37 | 0.48 | - | 45.52 | 5.30 | 0.91 | - | 24.37 | 85.21 | SbFeox | (Sb0.72Fe0.20Te0.08As0.02Au0.02Ag0.01)1.05O2.94 | |
| 8_11 | 1 | 4.11 | 52.68 | 1.99 | 1.54 | 5.64 | 12.35 | 1.19 | 4.88 | 1.12 | 9.3 | 94.8 | Auox | (Au0.89Ag0.06)0.95(Te0.32Fe0.23Se0.21Sb0.15S0.12As0.05Bi0.02)1.10O1.94 |
| 8_11 | 2 | 4.12 | 54.54 | 2.64 | 1.94 | 5.81 | 13.8 | 1.28 | 6.25 | 1.61 | 8.89 | 100.88 | Auox | (Au0.89Ag0.08)0.97(Te0.35Se0.26Fe0.23S0.16Sb0.15As0.06Bi0.03)1.24O1.79 |
| 8_11 | 3 | 4.23 | 53.55 | 2.50 | 1.98 | 5.8 | 11.84 | 1.58 | 5.39 | 1.31 | 9.25 | 97.43 | Auox | (Au0.89Ag0.08)0.97(Te0.35Se0.26Fe0.23S0.16Sb0.15As0.06Bi0.03)1.24O1.79 |
| 8_11 | 4 | 4.25 | 58.63 | 2.38 | 2.08 | 4.61 | 9.70 | 1.48 | 4.42 | 1.4 | 8.28 | 97.23 | Auox | (Au1.03Ag0.08)1.11(Te0.26Fe0.25Se0.19S0.15Sb0.13As0.07Bi0.03)1.08O1.80 |
| 8_11 | 5 | 2.71 | 50.98 | 2.39 | 1.51 | 2.9 | 20.88 | 0.98 | 8.59 | 1.91 | 5.89 | 98.74 | Auox | (Au0.97Ag0.08)1.05(Te0.61Se0.41S0.22Fe0.17Sb0.09As0.05Bi0.03)1.58O1.37 |
| 8_11 | 6 | 4.03 | 53.89 | 1.99 | 1.85 | 4.24 | 15.25 | 1.19 | 6.73 | 1.34 | 7.05 | 97.56 | Auox | (Au0.99Ag0.07)1.06(Te0.43Se0.31Fe0.25S0.15Sb0.13As0.06Bi0.03)1.36O1.59 |
| 8_11 | 7 | 4.47 | 53.75 | 1.42 | 2.07 | 7.21 | 12.97 | 1.37 | 5.63 | 1.45 | 9.03 | 99.37 | Auox | (Au0.89Ag0.04)0.93(Te0.33Fe0.25Se0.23Sb0.19S0.15As0.06Bi0.03)1.24O1.83 |
| 8_11 | 8 | 6.22 | 5.33 | 1.49 | - | 51.14 | 6.38 | 0.91 | - | 0.18 | 26.66 | 98.31 | SbFeox | (Sb0.73Fe0.18Te0.09As0.02 S0.01Au0.05Ag0.02)1.10O2.90 |
| 11_7 | 1 | 6.93 | 58.62 | 1.37 | - | 12.92 | 1.74 | 2.13 | - | - | 14.17 | 97.88 | Auox | (Au1.02Ag0.04)1.06(Fe0.40Sb0.36As0.10Te0.05)0.91O3.03 |
| 11_7 | 2 | 6.30 | 58.55 | 1.38 | - | 13.36 | 1.57 | 2.14 | - | - | 13.26 | 96.56 | Auox | (Au1.06Ag0.05)1.11(Sb0.39Fe0.38As0.10Te0.04)0.92O2.97 |
| 11_7 | 3 | - | 35.55 | - | 1.19 | - | 47.63 | - | 10.4 | 5.41 | - | 100.18 | mt | Au2.73Te5.64(S2.55Se1.99Bi0.09)4.63 |
| 11_7 | 4 | - | 36.77 | - | 0.74 | - | 47.39 | - | 7.92 | 4.89 | - | 97.71 | mt | Au2.98Te5.93(S2.43Se1.60Bi0.06)4.09 |
| 11_7 | 5 | - | 41.95 | - | - | - | 53.77 | - | 1.21 | - | - | 96.93 | calv | Au0.98(Te1.95Se0.07)2.02 |
| 11_7 | 6 | - | 44.06 | - | - | - | 54.28 | - | 1.16 | - | - | 99.50 | calv | Au1.01(Te1.92Se0.07)1.99 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolstykh, N.D.; Palyanova, G.A.; Bobrova, O.V.; Sidorov, E.G. Mustard Gold of the Gaching Ore Deposit (Maletoyvayam Ore Field, Kamchatka, Russia). Minerals 2019, 9, 489. https://doi.org/10.3390/min9080489
Tolstykh ND, Palyanova GA, Bobrova OV, Sidorov EG. Mustard Gold of the Gaching Ore Deposit (Maletoyvayam Ore Field, Kamchatka, Russia). Minerals. 2019; 9(8):489. https://doi.org/10.3390/min9080489
Chicago/Turabian StyleTolstykh, Nadezhda D., Galina A. Palyanova, Ol’ga V. Bobrova, and Evgeny G. Sidorov. 2019. "Mustard Gold of the Gaching Ore Deposit (Maletoyvayam Ore Field, Kamchatka, Russia)" Minerals 9, no. 8: 489. https://doi.org/10.3390/min9080489
APA StyleTolstykh, N. D., Palyanova, G. A., Bobrova, O. V., & Sidorov, E. G. (2019). Mustard Gold of the Gaching Ore Deposit (Maletoyvayam Ore Field, Kamchatka, Russia). Minerals, 9(8), 489. https://doi.org/10.3390/min9080489