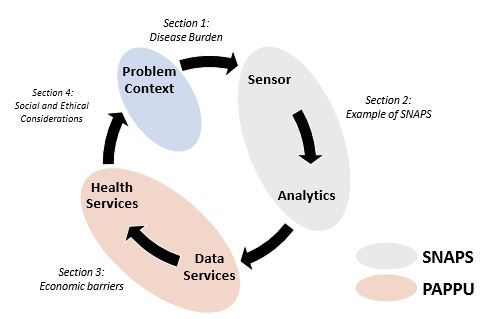
Sensor-as-a-Service: Convergence of Sensor Analytic Point Solutions (SNAPS) and Pay-A-Penny-Per-Use (PAPPU) Paradigm as a Catalyst for Democratization of Healthcare in Underserved Communities
Abstract
:1. Environmental Burden of Disease
2. Examples of SNAPS-ART
2.1. Early Assessment of Tuberculosis in Vulnerable Populations
2.2. Alerting Mercury Exposure in Artisanal Gold Mining Communities
3. Can We Overcome the Economic Barriers for Distributing Diagnostic Tools in Low-Income Settings?
4. Social and Ethical Considerations for the Development and Implementation of SNAPS
5. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Briggs, D. Environmental pollution and the global burden of disease. Br. Med. Bull. 2003, 68, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization and United Nations Environment Programme. Health and Environment: Tools for Effective Decision-Making: Review of Initial Findings; World Health Organization: Geneva, Switzerland; United Nations Environment Programme: Nairobi, Kenya, 2004. [Google Scholar]
- Cureton, S. Environmental victims: Environmental injustice issues that threaten the health of children living in poverty. Rev. Environ. Health 2011, 26, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Boutayeb, A. The double burden of communicable and non-communicable diseases in developing countries. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 191–199. [Google Scholar] [CrossRef]
- Gwatkin, D.R.; Bhuiya, A.; Victora, C.G. Making health systems more equitable. Lancet 2004, 364, 1273–1280. [Google Scholar] [CrossRef]
- Marmot, M. Social determinants of health inequalities. Lancet 2005, 365, 1099–1104. [Google Scholar] [CrossRef] [Green Version]
- Evans, G.W.; Kantrowitz, E. Socioeconomic status and health: The potential role of environmental risk exposure. Annu. Rev. Public Health 2002, 23, 303–331. [Google Scholar] [CrossRef] [Green Version]
- Waller, L.A.; Louis, T.A.; Carlin, B.P. Environmental justice and statistical summaries of differences in exposure distributions. J. Expo. Sci. Environ. Epidemiol. 1999, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- Mortality Rate, Infant (per 1,000 Live Births). Available online: https://data.worldbank.org/indicator/SP.DYN.IMRT.IN?end=2018&start=2018&type=points&view=bar (accessed on 27 September 2019).
- Moraga, P.; GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar]
- GBD 2016 Diarrhoeal Disease Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1211–1228. [Google Scholar] [CrossRef] [Green Version]
- Etchie, A.T.; Etchie, T.O.; Shen, H.; Pillarisetti, A.; Popovicheva, O. Burden of disease at the same limit of exposure to airborne polycyclic aromatic hydrocarbons varies significantly across countries depending on the gap in longevity. Ecotoxicol. Environ. Saf. 2019, 180, 420–429. [Google Scholar] [CrossRef]
- Schraufnagel, D.E.; Balmes, J.R.; Cowl, C.T.; De Matteis, S.; Jung, S.-H.; Mortimer, K.; Perez-Padilla, R.; Rice, M.B.; Riojas-Rodriguez, H.; Sood, A. Air pollution and noncommunicable diseases: A review by the Forum of International Respiratory Societies’ Environmental Committee, Part 2: Air pollution and organ systems. Chest 2019, 155, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Valdivia-Rivera, S.; Martínez-Cano, A.; Aguirre-García, G.; Lizardi-Jiménez, M. Hydrocarbon water-pollution related to chronic kidney disease in Tierra Blanca, a perfect storm. Environ. Int. 2018, 121, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Parihar, R.D.; Sharma, A.; Bakshi, P.; Sidhu, G.P.S.; Bali, A.S.; Karaouzas, I.; Bhardwaj, R.; Thukral, A.K.; Gyasi-Agyei, Y. Global evaluation of heavy metal content in surface water bodies: A meta-analysis using heavy metal pollution indices and multivariate statistical analyses. Chemosphere 2019, 236, 124364. [Google Scholar] [CrossRef] [PubMed]
- Johannson, K.A.; Balmes, J.R.; Collard, H.R. Air pollution exposure: A novel environmental risk factor for interstitial lung disease? Chest 2015, 147, 1161–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.-Y.; Gao, X.; Fang, Y.; Jiang, W.; Huang, K.; Hua, X.-G.; Yang, X.-J.; Chen, H.-B.; Jiang, Z.-X.; Zhang, X.-J. Human epidemiological evidence about the association between air pollution exposure and gestational diabetes mellitus: Systematic review and meta-analysis. Environ. Res. 2019, 180, 108843. [Google Scholar] [CrossRef]
- Stanaway, W.D.; GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar]
- Prüss-Ustün, A.; Wolf, J.; Bartram, J.; Clasen, T.; Cumming, O.; Freeman, M.C.; Gordon, B.; Hunter, P.R.; Medlicott, K.; Johnston, R. Burden of disease from inadequate water, sanitation and hygiene for selected adverse health outcomes: An updated analysis with a focus on low-and middle-income countries. Int. J. Hyg. Environ. Health 2019, 222, 765–777. [Google Scholar] [CrossRef]
- Ostro, B.; Spadaro, J.V.; Gumy, S.; Mudu, P.; Awe, Y.; Forastiere, F.; Peters, A. Assessing the recent estimates of the global burden of disease for ambient air pollution: Methodological changes and implications for low-and middle-income countries. Environ. Res. 2018, 166, 713–725. [Google Scholar] [CrossRef]
- Bauer, U.E.; Briss, P.A.; Goodman, R.A.; Bowman, B.A. Prevention of chronic disease in the 21st century: Elimination of the leading preventable causes of premature death and disability in the USA. Lancet 2014, 384, 45–52. [Google Scholar] [CrossRef]
- McLamore, E.S.; Datta, S.P.A.; Morgan, V.; Cavallaro, N.; Kiker, G.; Jenkins, D.M.; Rong, Y.; Gomes, C.; Claussen, J.; Vanegas, D.; et al. SNAPS: Sensor analytic point solutions for detection and decision support systems. Sensors 2019, 19, 4935. [Google Scholar] [CrossRef] [Green Version]
- From the lab to real-world use. Nat. Sustain. 2019, 2, 989. [CrossRef]
- Anderson, J.D., IV; Bagamian, K.H.; Muhib, F.; Amaya, M.P.; Laytner, L.A.; Wierzba, T.; Rheingans, R. Burden of enterotoxigenic Escherichia coli and shigella non-fatal diarrhoeal infections in 79 low-income and lower middle-income countries: A modelling analysis. Lancet Glob. Health 2019, 7, e321–e330. [Google Scholar] [CrossRef] [Green Version]
- Vélez-Torres, I.; Vanegas, D.C.; McLamore, E.S.; Hurtado, D. Mercury pollution and artisanal gold mining in Alto Cauca, Colombia: woman’s perception of health and environmental impacts. J. Environ. Dev. 2018, 27, 415–444. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Circular Economy and Health: Opportunities and Risk; World Health Organization Regional Office for Europe: Copenhagen, Denmark, 2018. [Google Scholar]
- Tuberculosis (TB). Available online: https://www.who.int/news-room/fact-sheets/detail/tuberculosis (accessed on 13 November 2019).
- Yang, T.; Zhong, J.; Zhang, J.; Li, C.; Yu, X.; Xiao, J.; Jia, X.; Ding, N.; Ma, G.; Wang, G. Pan-genomic study of Mycobacterium tuberculosis reflecting the primary/secondary genes, generality/individuality, and the interconversion through copy number variations. Front. Microbiol. 2018, 9, 1886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, H.M.Y.; Pai, M.; Raviglione, M.C. Ending tuberculosis in India: A political challenge & an opportunity. Indian J. Med. Res. 2018, 147, 217. [Google Scholar] [CrossRef] [PubMed]
- WHO|UN General Assembly High-Level Meeting on Ending TB. Available online: http://www.who.int/tb/features_archive/UNGA_HLM_ending_TB/en/ (accessed on 15 November 2019).
- Olaru, I.D.; Heyckendorf, J.; Grossmann, S.; Lange, C. Time to culture positivity and sputum smear microscopy during tuberculosis therapy. PLoS ONE 2014, 9, e106075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hobby, G.L.; Holman, A.P.; Iseman, M.D.; Jones, J.M. Enumeration of tubercle bacilli in sputum of patients with pulmonary tuberculosis. Antimicrob. Agents Chemother. 1973, 4, 94–104. [Google Scholar] [CrossRef] [Green Version]
- Desikan, P. Sputum smear microscopy in tuberculosis: Is it still relevant? Indian J. Med. Res. 2013, 137, 442. [Google Scholar]
- Steingart, K.R.; Ng, V.; Henry, M.; Hopewell, P.C.; Ramsay, A.; Cunningham, J.; Urbanczik, R.; Perkins, M.D.; Aziz, M.A.; Pai, M. Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: A systematic review. Lancet Infect. Dis. 2006, 6, 664–674. [Google Scholar] [CrossRef]
- Demers, A.-M.; Verver, S.; Boulle, A.; Warren, R.; Van Helden, P.; Behr, M.A.; Coetzee, D. High yield of culture-based diagnosis in a TB-endemic setting. BMC Infect. Dis. 2012, 12, 218. [Google Scholar] [CrossRef] [Green Version]
- Afsar, I.; Gunes, M.; Er, H.; Sener, A.G. Comparison of culture, microscopic smear and molecular methods in diagnosis of tuberculosis. Rev. Española Quimioter. 2018, 31, 435. [Google Scholar]
- Kik, S.V.; Denkinger, C.M.; Chedore, P.; Pai, M. Replacing smear microscopy for the diagnosis of tuberculosis: What is the market potential? Eur. Respir. J. 2014, 43, 1793–1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, V.; Nicol, M.; Tow, L. Sputum processing prior to Mycobacterium tuberculosis detection by culture or nucleic acid amplification testing: A narrative review. Res. Rev. J. Microbiol. Biotechnol. 2016, 5, 96–108. [Google Scholar]
- Das, S.; Narang, P.; Nagamiah, S.; Mishra, P.; Deotale, V.; Mendiratta, D. Evaluation of variants of carbol fuchsin solution to stain acid-fast bacilli in-situ by the pot method. Int. J. Tuberc. Lung Dis. 2015, 19, 1470–1475. [Google Scholar] [CrossRef]
- Ho, J.; Marks, G.; Fox, G. The impact of sputum quality on tuberculosis diagnosis: A systematic review. Int. J. Tuberc. Lung Dis. 2015, 19, 537–544. [Google Scholar] [CrossRef]
- Munir, M.; Shabbir, I.; Iqbal, R.; Khan, S.U. Comparison of detection of acid fast bacilli in clinical samples by AFB smear microscopy and culture in the diagnosis of tuberculosis in a tertiary care setting. Pak. J. Chest Med. 2015, 15, 1–8. [Google Scholar]
- Shea, Y.R.; Davis, J.L.; Huang, L.; Kovacs, J.A.; Masur, H.; Mulindwa, F.; Opus, S.; Chow, Y.; Murray, P.R. High sensitivity and specificity of acid-fast microscopy for diagnosis of pulmonary tuberculosis in an African population with a high prevalence of human immunodeficiency virus. J. Clin. Microbiol. 2009, 47, 1553–1555. [Google Scholar] [CrossRef] [Green Version]
- Cudahy, P.; Shenoi, S.V. Diagnostics for pulmonary tuberculosis. Postgrad. Med. J. 2016, 92, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Dunn, J.J.; Starke, J.R.; Revell, P.A. Laboratory diagnosis of Mycobacterium tuberculosis infection and disease in children. J. Clin. Microbiol. 2016, 54, 1434–1441. [Google Scholar] [CrossRef] [Green Version]
- Nyendak, M.R.; Lewinsohn, D.A.; Lewinsohn, D.M. New diagnostic methods for tuberculosis. Curr. Opin. Infect. Dis. 2009, 22, 174. [Google Scholar] [CrossRef] [Green Version]
- Ryu, Y.J. Diagnosis of pulmonary tuberculosis: Recent advances and diagnostic algorithms. Tuberc. Respir. Dis. 2015, 78, 64–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singhal, R.; Myneedu, V.P. Microscopy as a diagnostic tool in pulmonary tuberculosis. Int. J. Mycobacteriology 2015, 4, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engel, N.; Wachter, K.; Pai, M.; Gallarda, J.; Boehme, C.; Celentano, I.; Weintraub, R. Addressing the challenges of diagnostics demand and supply: Insights from an online global health discussion platform. BMJ Glob. Health 2016, 1, e000132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantoja, A.; Fitzpatrick, C.; Vassall, A.; Weyer, K.; Floyd, K. Xpert MTB/RIF for diagnosis of tuberculosis and drug-resistant tuberculosis: A cost and affordability analysis. Eur. Respir. J. 2013, 42, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Cazabon, D.; Pande, T.; Kik, S.; Van Gemert, W.; Sohn, H.; Denkinger, C.; Qin, Z.Z.; Waning, B.; Pai, M. Market penetration of Xpert MTB/RIF in high tuberculosis burden countries: A trend analysis from 2014–2016. Gates Open Res. 2018, 2. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, H.; Lee, S.; Ishizuka, A.; Hinoshita, E.; Hori, H.; Ishibashi, N.; Komada, K.; Norizuki, M.; Katsuma, Y.; Akashi, H. Challenges and opportunities for eliminating tuberculosis–leveraging political momentum of the UN high-level meeting on tuberculosis. BMC Public Health 2019, 19, 76. [Google Scholar] [CrossRef]
- Tanimura, T.; Jaramillo, E.; Weil, D.; Raviglione, M.; Lönnroth, K. Financial burden for tuberculosis patients in low-and middle-income countries: A systematic review. Eur. Respir. J. 2014, 43, 1763–1775. [Google Scholar] [CrossRef] [Green Version]
- Reed, J.L.; Basu, D.; Butzler, M.A.; McFall, S.M. XtracTB Assay, a Mycobacterium tuberculosis molecular screening test with sensitivity approaching culture. Sci. Rep. 2017, 7, 3653. [Google Scholar] [CrossRef] [Green Version]
- García-Basteiro, A.L.; DiNardo, A.; Saavedra, B.; Silva, D.; Palmero, D.; Gegia, M.; Migliori, G.B.; Duarte, R.; Mambuque, E.; Centis, R. Point of care diagnostics for tuberculosis. Pulmonology 2018, 24, 73–85. [Google Scholar] [CrossRef]
- Bhusal, N.; Shrestha, S.; Pote, N.; Alocilja, E. Nanoparticle-Based Biosensing of Tuberculosis, an Affordable and Practical Alternative to Current Methods. Biosensors 2019, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- Briceno, R.K.; Sergent, S.R.; Benites, S.M.; Alocilja, E.C. Nanoparticle-based Biosensing Assay for Universally Accessible Low-Cost TB Detection with Comparable Sensitivity as Culture. Diagnostics 2019, 9, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordillo-Marroquín, C.; Gómez-Velasco, A.; Sánchez-Pérez, H.; Pryg, K.; Shinners, J.; Murray, N.; Muñoz-Jiménez, S.; Bencomo-Alerm, A.; Gómez-Bustamante, A.; Jonapá-Gómez, L. Magnetic Nanoparticle-Based Biosensing Assay Quantitatively Enhances Acid-Fast Bacilli Count in Paucibacillary Pulmonary Tuberculosis. Biosensors 2018, 8, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Floyd, K.; Glaziou, P.; Houben, R.; Sumner, T.; White, R.; Raviglione, M. Global tuberculosis targets and milestones set for 2016–2035: Definition and rationale. Int. J. Tuberc. Lung Dis. 2018, 22, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, A.K.B.; Thomsen, J.F.; Mikkelsen, S. A review of mercury exposure among artisanal small-scale gold miners in developing countries. Int. Arch. Occup. Environ. Health 2014, 87, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Hentschel, T.; Hruschka, F.; Priester, M. Artisanal and Small-Scale Mining: Challenges and Opportunities, 1st ed.; Inernational Institute for Environment and Development (IIED) and World Business Council for Sustainable Development: London, UK, 2003. [Google Scholar]
- Cordy, P.; Veiga, M.M.; Salih, I.; Al-Saadi, S.; Console, S.; Garcia, O.; Mesa, L.A.; Velásquez-López, P.C.; Roeser, M. Mercury contamination from artisanal gold mining in Antioquia, Colombia: The world’s highest per capita mercury pollution. Sci. Total Environ. 2011, 410, 154–160. [Google Scholar] [CrossRef]
- United Nations Environment Programme. Global Mercury Assessment 2013: Sources, Emissions, Releases and Environmental Transport; UNEP Chemicals Branch: Geneva, Switzerland, 2013. [Google Scholar]
- Sengupta, P.; Banerjee, R.; Nath, S.; Das, S.; Banerjee, S. Metals and female reproductive toxicity. Hum. Exp. Toxicol. 2015, 34, 679–697. [Google Scholar] [CrossRef]
- Pizent, A.; Tariba, B.; Živković, T. Reproductive toxicity of metals in men. Arch. Ind. Hyg. Toxicol. 2012, 63, 35–46. [Google Scholar] [CrossRef]
- Matta, G.; Gjyli, L. Mercury, lead and arsenic: Impact on environment and human health. J. Chem. Pharm. Sci. 2016, 9, 718–725. [Google Scholar]
- Mahurpawar, M. Effects of heavy metals on human health. Int. J. Res. Granthaalayah 2015, 530, 1–7. [Google Scholar]
- Bridges, C.C.; Zalups, R.K. The aging kidney and the nephrotoxic effects of mercury. J. Toxicol. Environ. Health Part B 2017, 20, 55–80. [Google Scholar] [CrossRef]
- Ghaedi, M.; Reza Fathi, M.; Shokrollahi, A.; Shajarat, F. Highly selective and sensitive preconcentration of mercury ion and determination by cold vapor atomic absorption spectroscopy. Anal. Lett. 2006, 39, 1171–1185. [Google Scholar] [CrossRef]
- Yavuz, E.; Tokalıoğlu, Ş.; Patat, Ş. Magnetic dispersive solid phase extraction with graphene/ZnFe2O4 nanocomposite adsorbent for the sensitive determination of mercury in water and fish samples by cold vapor atomic absorption spectrometry. Microchem. J. 2018, 142, 85–93. [Google Scholar] [CrossRef]
- Jones, R.; Jacobson, M.; Jaffe, R.; West-Thomas, J.; Arfstrom, C.; Alli, A. Method development and sample processing of water, soil, and tissue for the analysis of total and organic mercury by cold vapor atomic Fluorescence spectrometry. Oceanogr. Lit. Rev. 1996, 2, 192. [Google Scholar]
- Yu, L.-P.; Yan, X.-P. Flow injection on-line sorption preconcentration coupled with cold vapor atomic fluorescence spectrometry and on-line oxidative elution for the determination of trace mercury in water samples. At. Spectrosc. 2004, 25, 145–153. [Google Scholar]
- Rodríguez-Reino, M.P.; Rodríguez-Fernández, R.; Peña-Vázquez, E.; Domínguez-González, R.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Mercury speciation in seawater by liquid chromatography-inductively coupled plasma-mass spectrometry following solid phase extraction pre-concentration by using an ionic imprinted polymer based on methyl-mercury–phenobarbital interaction. J. Chromatogr. A 2015, 1391, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; He, M.; Chen, B.; Deng, W.; Zheng, Q.; Hu, B. Magnetic solid phase extraction coupled with inductively coupled plasma mass spectrometry for the speciation of mercury in environmental water and human hair samples. Talanta 2016, 146, 93–99. [Google Scholar] [CrossRef]
- Barton, J.; García, M.B.G.; Santos, D.H.; Fanjul-Bolado, P.; Ribotti, A.; McCaul, M.; Diamond, D.; Magni, P. Screen-printed electrodes for environmental monitoring of heavy metal ions: A review. Microchim. Acta 2016, 183, 503–517. [Google Scholar] [CrossRef]
- Kabir, K.M.; Sabri, Y.M.; Lay, B.; Ippolito, S.J.; Bhargava, S.K. A silver electrode based surface acoustic wave (SAW) mercury vapor sensor: A physio-chemical and analytical investigation. Rsc Adv. 2016, 6, 36362–36372. [Google Scholar] [CrossRef]
- Lai, C.; Qin, L.; Zeng, G.; Liu, Y.; Huang, D.; Zhang, C.; Xu, P.; Cheng, M.; Qin, X.; Wang, M. Sensitive and selective detection of mercury ions based on papain and 2, 6-pyridinedicarboxylic acid functionalized gold nanoparticles. RSC Adv. 2016, 6, 3259–3266. [Google Scholar] [CrossRef]
- Noël, M.; Christensen, J.R.; Spence, J.; Robbins, C.T. Using laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) to characterize copper, zinc and mercury along grizzly bear hair providing estimate of diet. Sci. Total Environ. 2015, 529, 1–9. [Google Scholar] [CrossRef]
- Habte, G.; Hwang, I.M.; Kim, J.S.; Hong, J.H.; Hong, Y.S.; Choi, J.Y.; Nho, E.Y.; Jamila, N.; Khan, N.; Kim, K.S. Elemental profiling and geographical differentiation of Ethiopian coffee samples through inductively coupled plasma-optical emission spectroscopy (ICP-OES), ICP-mass spectrometry (ICP-MS) and direct mercury analyzer (DMA). Food Chem. 2016, 212, 512–520. [Google Scholar] [CrossRef]
- Cui, L.; Wu, J.; Ju, H. Electrochemical sensing of heavy metal ions with inorganic, organic and bio-materials. Biosens. Bioelectron. 2015, 63, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Bansod, B.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Huang, X.-J. Voltammetric determination of mercury (II). Trac Trends Anal. Chem. 2013, 51, 1–12. [Google Scholar] [CrossRef]
- Mauter, M.S.; Elimelech, M. Environmental applications of carbon-based nanomaterials. Environ. Sci. Technol. 2008, 42, 5843–5859. [Google Scholar] [CrossRef] [PubMed]
- Crevillen, A.G.; Escarpa, A.; García, C.D. Carbon-Based Nanomaterials in Analytical Chemistry, 1st ed.; Royal Society of Chemistry: London, UK, 2018. [Google Scholar]
- Xie, F.; Yang, M.; Jiang, M.; Huang, X.-J.; Liu, W.-Q.; Xie, P.-H. Carbon based nanomaterials-A promising electrochemical sensor toward persistent toxic substance. Trends Anal. Chem. 2019, 119, 115624. [Google Scholar] [CrossRef]
- Gong, J.; Zhou, T.; Song, D.; Zhang, L.; Hu, X. Stripping voltammetric detection of mercury (II) based on a bimetallic Au−Pt inorganic−organic hybrid nanocomposite modified glassy carbon electrode. Anal. Chem. 2009, 82, 567–573. [Google Scholar] [CrossRef]
- Ghanei-Motlagh, M.; Taher, M.A.; Heydari, A.; Ghanei-Motlagh, R.; Gupta, V.K. A novel voltammetric sensor for sensitive detection of mercury (II) ions using glassy carbon electrode modified with graphene-based ion imprinted polymer. Mater. Sci. Eng. 2016, 63, 367–375. [Google Scholar] [CrossRef]
- Pokhrel, L.R.; Ettore, N.; Jacobs, Z.L.; Zarr, A.; Weir, M.H.; Scheuerman, P.R.; Kanel, S.R.; Dubey, B. Novel carbon nanotube (CNT)-based ultrasensitive sensors for trace mercury (II) detection in water: A review. Sci. Total Environ. 2017, 574, 1379–1388. [Google Scholar] [CrossRef]
- Xing, H.; Xu, J.; Zhu, X.; Duan, X.; Lu, L.; Wang, W.; Zhang, Y.; Yang, T. Highly sensitive simultaneous determination of cadmium (II), lead (II), copper (II), and mercury (II) ions on N-doped graphene modified electrode. J. Electroanal. Chem. 2016, 760, 52–58. [Google Scholar] [CrossRef]
- Choi, S.-M.; Kim, D.-M.; Jung, O.-S.; Shim, Y.-B. A disposable chronocoulometric sensor for heavy metal ions using a diaminoterthiophene-modified electrode doped with graphene oxide. Anal. Chim. Acta 2015, 892, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-J.; Chen, X.; Yang, M. Persistent Toxic Substance Monitoring: Nanoelectrochemical Methods, 1st ed.; John Wiley & Sons: Weinheim, Germany, 2018. [Google Scholar]
- Duarte, K.; Justino, C.I.; Freitas, A.C.; Gomes, A.M.; Duarte, A.C.; Rocha-Santos, T.A. Disposable sensors for environmental monitoring of lead, cadmium and mercury. Trac Trends Anal. Chem. 2015, 64, 183–190. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 2014, 5, 5714. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, F.; Bavarian, B. Facile and scalable disposable sensor based on laser engraved graphene for electrochemical detection of glucose. Sci. Rep. 2016, 6, 27975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, P.; Kurra, N.; Xia, C.; Alshareef, H.N. Highly efficient laser scribed graphene electrodes for on-chip electrochemical sensing applications. Adv. Electron. Mater. 2016, 2, 160085. [Google Scholar] [CrossRef]
- Vanegas, D.; Patiño, L.; Mendez, C.; Oliveira, D.; Torres, A.; Gomes, C.; McLamore, E. Laser Scribed Graphene Biosensor for Detection of Biogenic Amines in Food Samples Using Locally Sourced Materials. Biosensors 2018, 8, 42. [Google Scholar] [CrossRef] [Green Version]
- Garland, N.T.; McLamore, E.S.; Cavallaro, N.D.; Mendivelso-Perez, D.; Smith, E.A.; Jing, D.; Claussen, J.C. Flexible Laser-Induced Graphene for Nitrogen Sensing in Soil. ACS Appl. Mater. Interfaces 2018, 10, 39124–39133. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Wang, J.; Chen, X.; Zhao, Q.; Ghaffar, A.; Chen, B. Application of graphene-based materials in water purification: From the nanoscale to specific devices. Environ. Sci. 2018, 5, 1264–1297. [Google Scholar] [CrossRef]
- Taguchi, M.; Schwalb, N.; Rong, Y.; Vanegas, D.; Garland, N.; Tan, M.; Yamaguchi, H.; Claussen, J.; McLamore, E. Pulsed: Pulsed sonoelectrodeposition of fractal nanoplatinum for enhancing amperometric biosensor performance. Analyst 2016, 141, 3367–3378. [Google Scholar] [CrossRef]
- Abdelbasir, S.; El-Sheikh, S.; Morgan, V.; Schmidt, H.; Casso-Hartmann, L.; Vanegas, D.; Velez-Torres, I.; McLamore, E. Graphene-anchored cuprous oxide nanoparticles from waste electric cables for electrochemical sensing. Sustain. Chem. Eng. 2018, 6, 12176–12186. [Google Scholar] [CrossRef]
- Jenkins, D.M.; Lee, B.E.; Jun, S.; Reyes-De-Corcuera, J.; McLamore, E.S. ABE-Stat, a Fully Open-Source and Versatile Wireless Potentiostat Project Including Electrochemical Impedance Spectroscopy. J. Electrochem. Soc. 2019, 166, B3056–B3065. [Google Scholar] [CrossRef]
- World Health Organization. Mercury in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- World Health Organization. Exposure to Mercury: A Major Public Health Concern. WHO, Public Health Environment International. 2007. Available online: https://www.who.int/ipcs/features/mercury.pdf (accessed on 20 November 2019).
- Taueg, C.; Sanfilippo, D.; Rowens, B.; Szejda, J.; Hesse, J. Acute and chronic poisoning from residential exposures to elemental mercury-michigan, 1989–1990. J. Toxicol. 1992, 30, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Bernhoft, R.A. Mercury toxicity and treatment: A review of the literature. J. Environ. Public Health 2012, 2012, 10. [Google Scholar] [CrossRef] [PubMed]
- Castilhos, Z.C.; Rodrigues-Filho, S.; Rodrigues, A.P.C.; Villas-Bôas, R.C.; Siegel, S.; Veiga, M.M.; Beinhoff, C. Mercury contamination in fish from gold mining areas in Indonesia and human health risk assessment. Sci. Total Environ. 2006, 368, 320–325. [Google Scholar] [CrossRef]
- Castilhos, Z.; Rodrigues-Filho, S.; Cesar, R.; Rodrigues, A.P.; Villas-Bôas, R.; de Jesus, I.; Lima, M.; Faial, K.; Miranda, A.; Brabo, E. Human exposure and risk assessment associated with mercury contamination in artisanal gold mining areas in the Brazilian Amazon. Environ. Sci. Pollut. Res. 2015, 22, 11255–11264. [Google Scholar] [CrossRef]
- Nakazawa, K.; Nagafuchi, O.; Kawakami, T.; Inoue, T.; Yokota, K.; Serikawa, Y.; Cyio, B.; Elvince, R. Human health risk assessment of mercury vapor around artisanal small-scale gold mining area, Palu city, Central Sulawesi, Indonesia. Ecotoxicol. Environ. Saf. 2016, 124, 155–162. [Google Scholar] [CrossRef]
- Petroczi, A.; Naughton, D. Mercury, cadmium and lead contamination in seafood: A comparative study to evaluate the usefulness of Target Hazard Quotients. Food Chem. Toxicol. 2009, 47, 298–302. [Google Scholar] [CrossRef]
- Telemedicine Predicted in 1925. Available online: https://www.smithsonianmag.com/history/telemedicine-predicted-in-1925-124140942/ (accessed on 20 November 2019).
- Arute, F.; Arya, K.; Babbush, R.; Bacon, D.; Bardin, J.C.; Barends, R.; Biswas, R.; Boixo, S.; Brandao, F.G.; Buell, D.A. Quantum supremacy using a programmable superconducting processor. Nature 2019, 574, 505–510. [Google Scholar] [CrossRef] [Green Version]
- Coase, R. The Nature of the Firm. Economica 1937, 4, 386–405. [Google Scholar] [CrossRef]
- Kay, N.M. Coase and the Contribution of ‘The Nature of the Firm’. Manag. Decis. Econ. 2015, 36, 44–54. [Google Scholar] [CrossRef]
- Kim, J.Y. Rich and Poor: Opportunities and Challenges in an Age of Disruption; World Bank: Washington, DC, USA, 2018. [Google Scholar] [CrossRef]
- Tonda, E.; Susan, C. Technology Challenges and tools for the implementation of the water-related sustainable development goals and targets. In Proceedings of the 2015 UN-Water Annual International Zaragoza Conference, Rio de Janeiro, Brazil, 15–17 January 2015. [Google Scholar]
- Banerjee, A.V.; Duflo, E. Poor Economics: A Radical Rethinking of the Way to Fight Global Poverty, 1st ed.; Public Affairs: New York, NY, USA, 2011. [Google Scholar]
- Mikami, M. Methodological divergence between coase and williamson in the history of transaction cost economics. Econ. J. Hokkaido Univ. 2011, 40, 41–57. [Google Scholar]
- Klein, S.; Frazier, G.L.; Roth, V.J. A transaction cost analysis model of channel integration in international markets. J. Mark. Res. 1990, 27, 196–208. [Google Scholar] [CrossRef]
- Pentland, A.; Fletcher, R.; Hasson, A. Daknet: Rethinking connectivity in developing nations. Computer 2004, 37, 78–83. [Google Scholar] [CrossRef]
- Martinez, A.; Villarroel, V.; Seoane, J.; del Pozo, F. Analysis of information and communication needs in rural primary health care in developing countries. IEEE Trans. Inf. Technol. Biomed. 2005, 9, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Pimenidis, E.; Sideridis, A.B.; Antonopoulou, E. Mobile devices and services: Bridging the digital divide in rural areas. Int. J. Electron. Secur. Digit. Forensics 2009, 2, 424–434. [Google Scholar] [CrossRef]
- James, J. Mechanisms of access to the Internet in rural areas of developing countries. Telemat. Inform. 2010, 27, 370–376. [Google Scholar] [CrossRef]
- Llanto, G.M.; Fukui, R. Innovations in Microfinance in Southeast Asia; PIDS Discussion Paper Series; No. 2003-11; Philippine Institute for Development Studies (PIDS): Makati City, Philippines, 2003. [Google Scholar]
- Nenova, T.; Niang, C.T. Bringing Finance to Pakistan’s Poor: Access to Finance for Small enterprises and the Underserved, 1st ed.; The World Bank: Washington, DC, USA, 2009. [Google Scholar]
- Bernardi, L.; Sarma, S.; Traub, K. The Inversion Factor: How to Thrive in the IoT Economy, 1st ed.; MIT Press: London, UK, 2017. [Google Scholar]
- Davis, F.D. A Technology Acceptance Model for Empirically Testing New End-User Information Systems: Theory and Results. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 20 December 1985. [Google Scholar]
- Rogers, E.M. A prospective and retrospective look at the diffusion model. J. Health Commun. 2004, 9, 13–19. [Google Scholar] [CrossRef]
- Williams, M.D.; Rana, N.P.; Dwivedi, Y.K. The unified theory of acceptance and use of technology (UTAUT): A literature review. J. Enterp. Inf. Manag. 2015, 28, 443–488. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.P.; Zheng, K.; Padman, R. Modeling the longitudinality of user acceptance of technology with an evidence-adaptive clinical decision support system. Decis. Support Syst. 2014, 57, 444–453. [Google Scholar] [CrossRef]
- Campbell, J.I.; Aturinda, I.; Mwesigwa, E.; Burns, B.; Haberer, J.E.; Bangsberg, D.R.; Holden, R.J.; Ware, N.C.; Siedner, M.J. The Technology Acceptance Model for Resource-Limited Settings (TAM-RLS): A novel framework for mobile health interventions targeted to low-literacy end-users in resource-limited settings. Aids Behav. 2017, 21, 3129–3140. [Google Scholar] [CrossRef]
- Sunny, S.; Patrick, L.; Rob, L. Impact of cultural values on technology acceptance and technology readiness. Int. J. Hosp. Manag. 2019, 77, 89–96. [Google Scholar] [CrossRef]
- Mathieson, K. Predicting user intentions: Comparing the technology acceptance model with the theory of planned behavior. Inf. Syst. Res. 1991, 2, 173–191. [Google Scholar] [CrossRef]
- Wallace, E.A.; Schumann, J.H.; Weinberger, S.E. Ethics of Commercial Screening Tests. Ann. Intern. Med. 2013, 158, 500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hersh, M. Technology Change, Technology Transfer and Ethics; University of Glasgow: Vienna, Austria, 2001. [Google Scholar]
- Street, J. Politics and Technology, 1st ed.; The Guilford Press: New York, NY, USA, 1992. [Google Scholar]
- Guidelines for Health Care Equipment Donations. Available online: https://www.who.int/medical_devices/publications/en/Donation_Guidelines.pdf (accessed on 5 November 2019).
- Perry, L.; Malkin, R. Effectiveness of medical equipment donations to improve health systems: How much medical equipment is broken in the developing world? Med. Biol. Eng. Comput. 2011, 49, 719–722. [Google Scholar] [CrossRef]
- Demirbas, A.; Groszman, K.; Pazmino, M.; Nolan, R.; Vanegas, D.C.; Welt, B.; Claussen, J.C.; Hondred, J.; McLamore, E.S. Cryoconcentration of bioflavonoid extract for enhanced photovoltaics and pH sensitive thin films. Biotechnol. Prog. 2018, 34, 206–217. [Google Scholar] [CrossRef]




| SSM Test | True TB Cases | Non-TB Cases | NCBA Test | True TB Cases | Non-TB Cases |
|---|---|---|---|---|---|
| Positive test | 32 | 0 | Positive test | 80 | 0 |
| Negative test | 48 | 420 | Negative test | 0 | 420 |
| Technique | Xpert MTB/RIF as the Gold Standard, % (95% CI) | ||||
|---|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | Accuracy | |
| SSM Test | 40 (29–52) | 100 (99–100) | 100 | 90 (88–91) | 90 (87–93) |
| NCBA Test | 100 (95–100) | 100 (99–100) | 100 | 100 | 100 (99–100) |
| Xpert MTB/RIF Categories ** | Very Low | Low | Medium | High | Total |
|---|---|---|---|---|---|
| Xpert MTB/RIF | 10 | 22 | 29 | 19 | 80 |
| NCBA | 10 | 22 | 29 | 19 | 80 |
| SSM | 0 | 3 | 14 | 15 | 32 |
| % Detection (NCBA/Xpert) | 100% | 100% | 100% | 100% | |
| % Detection (SSM/Xpert) | 0% | 14% | 48% | 79% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morgan, V.; Casso-Hartmann, L.; Bahamon-Pinzon, D.; McCourt, K.; Hjort, R.G.; Bahramzadeh, S.; Velez-Torres, I.; McLamore, E.; Gomes, C.; Alocilja, E.C.; et al. Sensor-as-a-Service: Convergence of Sensor Analytic Point Solutions (SNAPS) and Pay-A-Penny-Per-Use (PAPPU) Paradigm as a Catalyst for Democratization of Healthcare in Underserved Communities. Diagnostics 2020, 10, 22. https://doi.org/10.3390/diagnostics10010022
Morgan V, Casso-Hartmann L, Bahamon-Pinzon D, McCourt K, Hjort RG, Bahramzadeh S, Velez-Torres I, McLamore E, Gomes C, Alocilja EC, et al. Sensor-as-a-Service: Convergence of Sensor Analytic Point Solutions (SNAPS) and Pay-A-Penny-Per-Use (PAPPU) Paradigm as a Catalyst for Democratization of Healthcare in Underserved Communities. Diagnostics. 2020; 10(1):22. https://doi.org/10.3390/diagnostics10010022
Chicago/Turabian StyleMorgan, Victoria, Lisseth Casso-Hartmann, David Bahamon-Pinzon, Kelli McCourt, Robert G. Hjort, Sahar Bahramzadeh, Irene Velez-Torres, Eric McLamore, Carmen Gomes, Evangelyn C. Alocilja, and et al. 2020. "Sensor-as-a-Service: Convergence of Sensor Analytic Point Solutions (SNAPS) and Pay-A-Penny-Per-Use (PAPPU) Paradigm as a Catalyst for Democratization of Healthcare in Underserved Communities" Diagnostics 10, no. 1: 22. https://doi.org/10.3390/diagnostics10010022
APA StyleMorgan, V., Casso-Hartmann, L., Bahamon-Pinzon, D., McCourt, K., Hjort, R. G., Bahramzadeh, S., Velez-Torres, I., McLamore, E., Gomes, C., Alocilja, E. C., Bhusal, N., Shrestha, S., Pote, N., Briceno, R. K., Datta, S. P. A., & Vanegas, D. C. (2020). Sensor-as-a-Service: Convergence of Sensor Analytic Point Solutions (SNAPS) and Pay-A-Penny-Per-Use (PAPPU) Paradigm as a Catalyst for Democratization of Healthcare in Underserved Communities. Diagnostics, 10(1), 22. https://doi.org/10.3390/diagnostics10010022