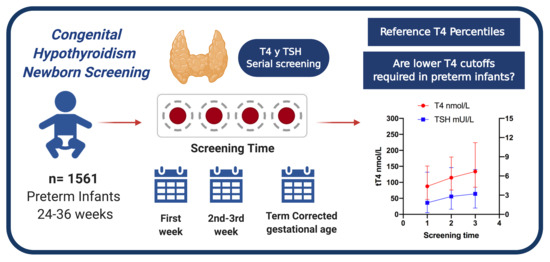
Reference Percentiles and Changes over Time for Total Thyroxine in Preterm Infants: A Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Screening Methods
2.3. Laboratory Methods
2.4. Data Collection
2.5. Statistical Analysis
Comparisons of tT4 and TSH between Gestational Age Groups and Screening Times
3. Results
3.1. Newborn Screening Results
3.2. Infants Included for tT4 Percentiles
Serial Screening Samples for tT4 Percentiles
3.3. Primary Outcome: tT4 Percentiles
3.4. Comparisons of tT4 between Gestational Age Groups
3.5. Comparisons of tT4 between Screening Times
3.6. Comparisons of TSH between Screening Times
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grüters, A.; Krude, H. Detection and treatment of congenital hypothyroidism. Nat. Rev. Endocrinol. 2012, 8, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Ford, G.; LaFranchi, S.H. Screening for congenital hypothyroidism: A worldwide view of strategies. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; van Trotsenburg, A.S.P.; Schoenmakers, N. Diagnosis of Endocrine Disease: Congenital hypothyroidism: Update and perspectives. Eur. J. Endocrinol. 2018, 179, R297–R317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaluarachchi, D.C.; Allen, D.B.; Eickhoff, J.C.; Dawe, S.J.; Baker, M.W. Increased Congenital Hypothyroidism Detection in Preterm Infants with Serial Newborn Screening. J. Pediatr. 2019, 207, 220–225. [Google Scholar] [CrossRef]
- Braslavsky, D.; Méndez, M.V.; Prieto, L.; Keselman, A.; Enacan, R.; Gruñeiro-Papendieck, L.; Jullien, N.; Savenau, A.; Reynaud, R.; Brue, T.; et al. Pilot Neonatal Screening Program for Central Congenital Hypothyroidism: Evidence of Significant Detection. Horm. Res. Paediatr. 2017, 88, 274–280. [Google Scholar] [CrossRef]
- Lak, R.; Yazdizadeh, B.; Davari, M.; Nouhi, M.; Kelishadi, R. Newborn Screening for Galactosaemia. Cochrane Database of Systematic Reviews. 2016. Available online: http://dx.doi.org/10.1002/14651858.cd012272 (accessed on 13 December 2019).
- Simpson, J.; Williams, F.L.R.; Delahunty, C.; van Toor, H.; Wu, S.-Y.; Ogston, S.A.; Visser, T.J.; Hume, R. Serum thyroid hormones in preterm infants and relationships to indices of severity of intercurrent illness. J. Clin. Endocrinol. Metab. 2005, 90, 1271–1279. [Google Scholar] [CrossRef]
- Findley, T.O.; Shah, A.; Bell, C.; Khan, A. The value of serial newborn screening for congenital hypothyroidism using thyroxine (T4) in the neonatal intensive care unit. J. Perinatol. 2019, 39, 1065–1071. [Google Scholar] [CrossRef]
- McGrath, N.; Hawkes, C.P.; Mayne, P.; Murphy, N.P. Optimal Timing of Repeat Newborn Screening for Congenital Hypothyroidism in Preterm Infants to Detect Delayed Thyroid-Stimulating Hormone Elevation. J. Pediatr. 2019, 205, 77–82. [Google Scholar] [CrossRef]
- Hashemipour, M.; Hovsepian, S.; Ansari, A.; Keikha, M.; Khalighinejad, P.; Niknam, N. Screening of congenital hypothyroidism in preterm, low birth weight and very low birth weight neonates: A systematic review. Pediatr. Neonatol. 2018, 59, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Kaluarachchi, D.C.; Allen, D.B.; Eickhoff, J.C.; Dawe, S.J.; Baker, M.W. Thyroid-Stimulating Hormone Reference Ranges for Preterm Infants. Pediatrics 2019, 144, e20190290. [Google Scholar] [CrossRef] [Green Version]
- Prevention, Screening and Diagnosis of Congenital Hypothyroidism in the First Level of Care. Practice Guideline CENETEC, Mexico. 2015. Available online: http://www.cenetec-difusion.com/CMGPC/ISSSTE-135-08/ER.pdf (accessed on 5 January 2020).
- American Academy of Pediatrics Committee on Fetus and Newborn. Levels of neonatal care. Pediatrics 2012, 130, 587–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, N.; Hume, R.; van Toor, H.; Matthews, T.G.; Ogston, S.A.; Wu, S.-Y.; Visser, T.J.; Williams, F.L. The hypothalamic-pituitary-thyroid axis in preterm infants; changes in the first 24 hours of postnatal life. J. Clin. Endocrinol. Metab. 2004, 89, 2824–2831. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.E.; Faix, J.E.; Hermos, R.J.; Mullaney, D.M.; Rojan, D.A.; Mitchell, M.L.; Klein, R.Z. Thyroid function in very low birth weight infants: Effects on neonatal hypothyroidism screening. J. Pediatr. 1996, 128, 548–554. [Google Scholar] [CrossRef]
- Sharma, J.D.; Nazir, M.F.H.; Khan, A.G.; Hoque, B. Does Hypothyroxinemia of Preterm Neonates Persist beyond 7 weeks of Life? Indian J. Pediatr. 2019, 86, 686–691. [Google Scholar] [CrossRef]
- Dembinski, J.; Arpe, V.; Kroll, M.; Bartmann, P.; Hieronimi, G. Thyroid Function in Healthy and Sick Very-Low-Birth-Weight Infants—Thyrotropin and Free Thyroxine Levels until the Sixth Week of Age. Neonatology 2001, 80, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Torkaman, M.; Ghasemi, F.; Amirsalari, S.; Abyazi, M.; Afsharpaiman, S.; Kavehmanesh, Z.; Beiraghdar, F.; Saburi, A. Thyroid Function Test in Pre-term Neonates During the First Five Weeks of Life. Int. J. Prev. Med. 2013, 4, 1271–1276. [Google Scholar] [PubMed]
- Williams, F.L.R.; Simpson, J.; Delahunty, C.; Ogston, S.A.; Bongers-Schokking, J.J.; Murphy, N.; van Toor, H.; Wu, S.Y.; Visser, T.J.; Hume, R.; et al. Developmental trends in cord and postpartum serum thyroid hormones in preterm infants. J. Clin. Endocrinol. Metab. 2004, 89, 5314–5320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hijman, A.-I.; Konrad, D.; Fingerhut, R. Determining Reference Ranges for Total T4 in Dried Blood Samples for Newborn Screening. Int. J. Neonatal Screen. 2020, 6, 17. [Google Scholar] [CrossRef] [Green Version]
- Cavarzere, P.; Camilot, M.; Popa, F.I.; Lauriola, S.; Teofoli, F.; Gaudino, R.; Vincenzi, M.; Antoniazzi, F. Congenital hypothyroidism with delayed TSH elevation in low-birth-weight infants: Incidence, diagnosis and management. Eur. J. Endocrinol. 2016, 175, 395–402. [Google Scholar] [CrossRef] [Green Version]
- Uchiyama, A.; Kushima, R.; Watanabe, T.; Kusuda, S. Effect of l-thyroxine supplementation on infants with transient hypothyroxinemia of prematurity at 18 months of corrected age: Randomized clinical trial. J. Pediatr. Endocrinol. Metab. 2015, 28, 177–182. [Google Scholar] [CrossRef]
- Hollanders, J.J.; Israëls, J.; van der Pal, S.M.; Verkerk, P.H.; Rotteveel, J.; Finken, M.J.J.; Dutch POPS-19 Collaborative Study Group. No Association Between Transient Hypothyroxinemia of Prematurity and Neurodevelopmental Outcome in Young Adulthood. J. Clin. Endocrinol. Metab. 2015, 100, 4648–4653. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.O.; Tan, M.G.; Poon, W.B. Correction: Lack of association between hypothyroxinemia of prematurity and transient thyroid abnormalities with adverse long term neurodevelopmental outcome in very low birth weight infants. PLoS ONE 2019, 14, e0223867. [Google Scholar] [CrossRef] [PubMed]
- Korzeniewski, S.J.; Grigorescu, V.; Kleyn, M.; Young, W.; Birbeck, G.L.; Todem, D.; Romero, R.; Chaiworapongsa, T.; Paneth, N. Performance metrics after changes in screening protocol for congenital hypothyroidism. Pediatrics 2012, 130, e1252–e1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connelly, K.J.; Pierce, M.J.; Hanna, C.; LaFranchi, S.H. Detecting Congenital Central Hypothyroidism by Newborn Screening: Difficulty in Distinguishing from Congenital Thyroxine-Binding Globulin Deficiency. Horm. Res. Paediatr. 2017, 88, 331–338. [Google Scholar] [CrossRef]
- Korada, M.; Pearce, M.S.; Ward Platt, M.P.; Avis, E.; Turner, S.; Wastell, H.; Cheetham, T. Repeat testing for congenital hypothyroidism in preterm infants is unnecessary with an appropriate thyroid stimulating hormone threshold. Arch. Dis. Child. Fetal Neonatal Ed. 2008, 93, F286–F288. [Google Scholar] [CrossRef]
- Zamboni, G.; Zaffanello, M.; Rigon, F.; Radetti, G.; Gaudino, R.; Tatò, L. Diagnostic effectiveness of simultaneous thyroxine and thyroid-stimulating hormone screening measurements. Thirteen years’ experience in the Northeast Italian Screening Programme. J. Med. Screen. 2004, 11, 8–10. [Google Scholar] [CrossRef]



| Characteristics | n = 1561 | |
|---|---|---|
| Gender, Male, n (%) | 787 (50.4) | |
| Gestational age, n (%) | ||
| 24–27 weeks | 37 (2.4) | |
| 28–30 weeks | 146 (9.4) | |
| 31–34 weeks | 519 (33.2) | |
| 35–36 weeks | 859 (55) | |
| Birth weight, n (%) | ||
| <1000 g | 95 (6.1) | |
| 1001–1500 g | 232 (14.9) | |
| 1501–2000 g | 379 (24.2) | |
| 2001–2500 g | 472 (30.2) | |
| >2500 g | 384 (24.6) | |
| Days of life at screening, median (IQR) * | ||
| First screening | 3 (0) | |
| Second screening | 18 (1) | |
| Third screening | 41 (22) | |
| Maternal history, n (%) | ||
| Thyroid disease | 125 (8) | |
| Multiple pregnancies | 390 (25) | |
| Mode of delivery, n (%) | ||
| Vaginal | 270 (17.3) | |
| Cesarean section | 1291 (82.7) | |
| Congenital malformations, n (%) | 79 (5.1) | |
| Gestational Age (Weeks) | n | 5th | 10th | 25th | 50th | 75th | 90th | 95th | Units |
|---|---|---|---|---|---|---|---|---|---|
| First screening: week 1, n = 1549 | |||||||||
| 24–27 | 35 | 2.6 | 3.7 | 4.8 | 5.9 | 7.0 | 8.9 | 9.5 | μg/dL |
| 33.4 | 47.6 | 61.7 | 75.9 | 90.1 | 114.5 | 122.2 | nmol/L | ||
| 28–30 | 146 | 3.6 | 4.4 | 5.7 | 6.8 | 8.8 | 10.4 | 11.7 | μg/dL |
| 46.3 | 56.6 | 73.3 | 87.5 | 113.2 | 133.8 | 150.6 | nmol/L | ||
| 31–34 | 514 | 5.7 | 6.4 | 8.3 | 10.3 | 12.5 | 15.0 | 16.9 | μg/dL |
| 73.3 | 82.3 | 106.8 | 132.5 | 160.9 | 193.1 | 217.5 | nmol/L | ||
| 35–36 | 854 | 8.1 | 9.1 | 10.7 | 12.8 | 15.1 | 17.6 | 19.7 | μg/dL |
| 104.2 | 117.1 | 137.7 | 164.7 | 194.3 | 226.5 | 253.5 | nmol/L | ||
| Second screening: week 2–3, n = 1430 | |||||||||
| 24–27 | 36 | 4.0 | 5.3 | 7.0 | 8.1 | 9.7 | 11.0 | 12.0 | μg/dL |
| 51.4 | 68.2 | 90.1 | 104.2 | 124.8 | 141.5 | 154.4 | nmol/L | ||
| 28–30 | 143 | 5.9 | 6.4 | 7.4 | 8.9 | 10.8 | 12.6 | 13.9 | μg/dL |
| 75.9 | 82.3 | 95.2 | 114.5 | 139 | 162.1 | 178.9 | nmol/L | ||
| 31–34 | 494 | 6.4 | 7.1 | 8.4 | 9.8 | 11.7 | 14.0 | 15.5 | μg/dL |
| 82.3 | 91.4 | 108.1 | 126.1 | 150.6 | 180.2 | 199.5 | nmol/L | ||
| 35–36 | 757 | 6.7 | 7.3 | 8.6 | 10.1 | 12 | 14.2 | 16 | μg/dL |
| 86.2 | 93.9 | 110.6 | 130 | 154.4 | 182.7 | 205.9 | nmol/L | ||
| Third screening: Term-corrected gestational age (38 weeks of gestation), n = 571 | |||||||||
| 24–27 | 32 | 5.8 | 7.6 | 8.4 | 9.9 | 12.6 | 14.5 | 16.0 | μg/dL |
| 74.6 | 97.8 | 108.1 | 127.4 | 162.1 | 186.6 | 205.9 | nmol/L | ||
| 28–30 | 117 | 6.5 | 7.0 | 8.6 | 10.4 | 12.3 | 15.1 | 17.4 | μg/dL |
| 83.6 | 90.1 | 110.6 | 133.8 | 158.3 | 194.3 | 223.9 | nmol/L | ||
| 31–34 | 422 | 6.2 | 6.8 | 8.2 | 10.1 | 12.5 | 14.7 | 16.3 | μg/dL |
| 79.8 | 87.5 | 105.5 | 130 | 160.9 | 189.2 | 209.7 | nmol/L | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Robles, C.M.; Roldan-Valadez, E.; Martínez-Cruz, N.; Arce-Sánchez, L.; Priego-Zurita, A.L.; Estrada-Gutierrez, G.; Reyes-Muñoz, E. Reference Percentiles and Changes over Time for Total Thyroxine in Preterm Infants: A Retrospective Cohort Study. Diagnostics 2020, 10, 475. https://doi.org/10.3390/diagnostics10070475
Flores-Robles CM, Roldan-Valadez E, Martínez-Cruz N, Arce-Sánchez L, Priego-Zurita AL, Estrada-Gutierrez G, Reyes-Muñoz E. Reference Percentiles and Changes over Time for Total Thyroxine in Preterm Infants: A Retrospective Cohort Study. Diagnostics. 2020; 10(7):475. https://doi.org/10.3390/diagnostics10070475
Chicago/Turabian StyleFlores-Robles, Claudia M., Ernesto Roldan-Valadez, Nayeli Martínez-Cruz, Lidia Arce-Sánchez, Ana L. Priego-Zurita, Guadalupe Estrada-Gutierrez, and Enrique Reyes-Muñoz. 2020. "Reference Percentiles and Changes over Time for Total Thyroxine in Preterm Infants: A Retrospective Cohort Study" Diagnostics 10, no. 7: 475. https://doi.org/10.3390/diagnostics10070475
APA StyleFlores-Robles, C. M., Roldan-Valadez, E., Martínez-Cruz, N., Arce-Sánchez, L., Priego-Zurita, A. L., Estrada-Gutierrez, G., & Reyes-Muñoz, E. (2020). Reference Percentiles and Changes over Time for Total Thyroxine in Preterm Infants: A Retrospective Cohort Study. Diagnostics, 10(7), 475. https://doi.org/10.3390/diagnostics10070475