A Broader Perspective on Anti-Ro Antibodies and Their Fetal Consequences—A Case Report and Literature Review
Abstract
:1. Introduction
1.1. What Exactly Do the Anti-Ro/SSA and Anti-La/SSB Antigen–Antibody Systems Represent?
1.2. Preferred Methods in the Detection of Anti-Ro and Anti-La Antibodies
1.3. Autoimmune Disorders with Detectable Anti-Ro Antibodies
1.4. Who Should Be Tested for the Presence of Anti-Ro Antibodies?
2. Pregnancy in Women with Anti-Ro/SSA Antibodies
2.1. Frequency of Gestational Complications
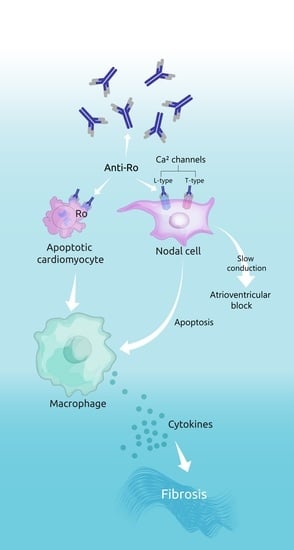
2.2. Molecular Mechanisms Leading to Fetal Complications
2.2.1. The “Apoptosis Hypothesis”
2.2.2. The “Calcium Channel Hypothesis”
2.3. Is There a Relationship between the Titers of Anti-Ro/SSA and/or Anti-La/SSB Antibodies and the Incidence of Congenital Heart Block?
2.4. Clinical Manifestations of Neonatal Lupus
2.4.1. Cutaneous Manifestations
2.4.2. Cardiac Manifestations
2.4.3. Other Transient and more Rare Manifestations
2.5. Manifestations That Suggest the Diagnosis of Neonatal Lupus
3. Management of Positive Anti-Ro/SSA Pregnancies
3.1. First Pregnancy in Asymptomatic Carriers of Anti-Ro/SSA Antibodies
3.2. In Utero Management of Neonatal Lupus
3.3. Postnatal Management of Neonatal Lupus
3.4. Prevention of Neonatal Lupus in Subsequent Pregnancies
4. Case Report
5. Discussion
5.1. The Rheumatologists’ Point of View
5.2. The Maternal–Fetal Specialist Point of View
5.3. The Cardiologist Point of View
5.3.1. Short-Term Management
5.3.2. Long-Term Consequences and Follow-Up
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Deutscher, S.L.; Harley, J.B.; Keene, J.D. Molecular analysis of the 60-kDa human Ro ribonucleoprotein. Proc. Natl. Acad. Sci. USA 1988, 85, 9479–9483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, D.; Shi, H.; Smith, J.D.; Chen, X.; Noe, D.A.; Cedervall, T.; Yang, D.D.; Eynon, E.; Brash, D.E.; Kashgarian, M.; et al. A lupus-like syndrome develops in mice lacking the Ro 60-kDa protein, a major lupus autoantigen. Proc. Natl. Acad. Sci. USA 2003, 100, 7503–7508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, E.K.; Hamel, J.C.; Buyon, J.P.; Tan, E.M. Molecular definition and sequence motifs of the 52-kD component of human SS-A/Ro autoantigen. J. Clin. Investig. 1991, 87, 68–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgs, R.; Lazzari, E.; Wynne, C.; Gabhann, J.N.; Espinosa, A.; Wahren-Herlenius, M.; Jefferies, C.A. Self protection from anti-viral responses—Ro52 promotes degradation of the transcription factor IRF7 downstream of the viral toll-like receptors. PLoS ONE 2010, 5, e11776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgs, R.; Gabhann, J.N.; Larbi, N.B.; Breen, E.P.; Fitzgerald, K.A.; Jefferies, C.A. The E3 ubiquitin ligase Ro52 negatively regulates IFN-β production post-pathogen recognition by polyubiquitin-mediated degradation of IRF3. J. Immunol. 2008, 181, 1780–1786. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Niida, M.; Tanaka, M.; Kamitani, T. Ro52-mediated monoubiquitination of IKKβ down-regulates NF-κB signalling. J. Biochem. 2009, 146, 821–832. [Google Scholar] [CrossRef] [Green Version]
- Bolland, S.; Garcia-Sastre, A. Vicious circle: Systemic autoreactivity in Ro52/TRIM21-deficient mice. J. Exp. Med. 2009, 206, 1647–1651. [Google Scholar] [CrossRef]
- Eftekhari, P.; Sallé, L.; Lezoualc’h, F.; Mialet, J.; Gastineau, M.; Briand, J.P.; Isenberg, D.A.; Fournié, G.J.; Argibay, J.; Fischmeister, R.; et al. Anti-SSA/Ro52 autoantibodies blocking the cardiac 5-HT4 serotoninergic receptor could explain neonatal lupus congenital heart block. Eur. J. Immunol. 2000, 30, 2782–2790. [Google Scholar] [CrossRef]
- Fok, V.; Friend, K.; Steitz, J.A. Epstein-Barr virus noncoding RNAs are confined to the nucleus, whereas their partner, the human La protein, undergoes nucleocytoplasmic shuttling. J. Cell Biol. 2006, 173, 319–325. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, H.; Tian, H.; Liang, C.; Chen, S.; Liu, Q. Autoantigen la promotes efficient RNAi, antiviral response, and transposon silencing by facilitating multiple-turnover RISC catalysis. Mol. Cell 2011, 44, 502–508. [Google Scholar] [CrossRef] [Green Version]
- Solomon, D.H.; Kavanaugh, A.J.; Schur, P.H. Evidence-based guidelines for the use of immunologic tests: Antinuclear antibody testing. Arthritis Rheum. 2002, 47, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Agmon-Levin, N.; Damoiseaux, J.; Kallenberg, C.; Sack, U.; Witte, T.; Herold, M.; Bossuyt, X.; Musset, L.; Cervera, R.; Plaza-Lopez, A.; et al. International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Ann. Rheum. Dis. 2014, 73, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Lazzaroni, M.G.; Dall’Ara, F.; Fredi, M.; Nalli, C.; Reggia, R.; Lojacono, A.; Ramazzotto, F.; Zatti, S.; Andreoli, L.; Tincani, A. A comprehensive review of the clinical approach to pregnancy and systemic lupus erythematosus. J. Autoimmun. 2016, 74, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidis, N.C.; Kapsogeorgou, E.K.; Tzioufas, A.G. A comprehensive review of autoantibodies in primary Sjögren’s syndrome: Clinical phenotypes and regulatory mechanisms. J. Autoimmun. 2014, 51, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Retamozo, S.; Akasbi, M.; Brito-Zerón, P.; Bosch, X.; Bove, A.; Perez-de-Lis, M.; Jimenez, I.; Soto-Cardenas, M.J.; Gandía, M.; Diaz-Lagares, C.; et al. Anti-Ro52 antibody testing influences the classification and clinical characterisation of primary Sjögren’s syndrome. Clin. Exp. Rheumatol. 2012, 30, 686–692. [Google Scholar] [PubMed]
- Hudson, M.; Pope, J.; Mahler, M.; Tatibouet, S.; Steele, R.; Baron, M.; Fritzler, M.J.; Canadian Scleroderma Research Group. Clinical significance of antibodies to Ro52/TRIM21 in systemic sclerosis. Arthritis Res. Ther. 2012, 14, R50. [Google Scholar] [CrossRef] [Green Version]
- Gunnarsson, R.; El-Hage, F.; Aaløkken, T.M.; Reiseter, S.; Lund, M.B.; Garen, T.; Molberg, Ø.; Norwegian MCTD Study Group. Associations between anti-Ro52 antibodies and lung fibrosis in mixed connective tissue disease. Rheumatology 2016, 55, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017, 69, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.P.; Wang, C.G.; Liu, X.; Huang, Y.Q.; Guo, D.L.; Jing, X.Z.; Yuan, C.G.; Yang, S.; Liu, J.M.; Han, M.S.; et al. The prevalence of antinuclear antibodies in the general population of china: A cross-sectional study. Curr. Ther. Res. Clin. Exp. 2014, 76, 116–119. [Google Scholar] [CrossRef] [Green Version]
- Satoh, M.; Chan, E.K.; Ho, L.A.; Rose, K.M.; Parks, C.G.; Cohn, R.D.; Jusko, T.A.; Walker, N.J.; Germolec, D.R.; Whitt, I.Z.; et al. Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum. 2012, 64, 2319–2327. [Google Scholar] [CrossRef]
- Hayashi, N.; Koshiba, M.; Nishimura, K.; Sugiyama, D.; Nakamura, T.; Morinobu, S.; Kawano, S.; Kumagai, S. Prevalence of disease-specific antinuclear antibodies in general population: Estimates from annual physical examinations of residents of a small town over a 5-year period. Mod. Rheumatol. 2008, 18, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Arbuckle, M.R.; McClain, M.T.; Rubertone, M.V.; Scofield, R.H.; Dennis, G.J.; James, J.A.; Harley, J.B. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N. Engl. J. Med. 2003, 349, 1526–1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theander, E.; Jonsson, R.; Sjöström, B.; Brokstad, K.; Olsson, P.; Henriksson, G. Prediction of Sjögren’s syndrome years before diagnosis and identification of patients with early onset and severe disease course by autoantibody profiling. Arthritis Rheumatol. 2015, 67, 2427–2436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sammaritano, L.R.; Bermas, B.L.; Chakravarty, E.E.; Chambers, C.; Clowse, M.E.; Lockshin, M.D.; Marder, W.; Guyatt, G.; Branch, D.W.; Buyon, J.; et al. 2020 American College of Rheumatology Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases. Arthritis Care Res. 2020, 72, 461–488. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, B.; Bhan, R.; Trad, C.; Cohen, R.; Saxena, A.; Buyon, J.; Izmirly, P. Autoimmune-mediated congenital heart block. In Best Practice and Research: Clinical Obstetrics and Gynaecology; Bailliere Tindall Ltd.: London, UK, 2019. [Google Scholar]
- Izmirly, P.M.; Saxena, A.; Kim, M.Y.; Wang, D.; Sahl, S.K.; Llanos, C.; Friedman, D.; Buyon, J.P. Maternal and fetal factors associated with mortality and morbidity in a multi-racial/ethnic registry of anti-SSA/Ro-associated cardiac neonatal lupus. Circulation 2011, 124, 1927–1935. [Google Scholar] [CrossRef] [Green Version]
- Lopes, L.M.; Tavares, G.M.; Damiano, A.P.; Lopes, M.A.; Aiello, V.D.; Schultz, R.; Zugaib, M. Perinatal outcome of fetal atrioventricular block one-hundred-sixteen cases from a single institution. Circulation 2008, 118, 1268–1275. [Google Scholar] [CrossRef] [Green Version]
- Eronen, M.; Sirèn, M.K.; Ekblad, H.; Tikanoja, T.; Julkunen, H.; Paavilainen, T. Short- and long-term outcome of children with congenital complete heart block diagnosed in utero or as a newborn. Pediatrics 2000, 106, 86–91. [Google Scholar] [CrossRef]
- Eliasson, H.; Sonesson, S.E.; Sharland, G.; Granath, F.; Simpson, J.M.; Carvalho, J.S.; Jicinska, H.; Tomek, V.; Dangel, J.; Zielinsky, P.; et al. Isolated atrioventricular block in the fetus: A retrospective, multinational, multicenter study of 175 patients. Circulation 2011, 124, 1919–1926. [Google Scholar] [CrossRef] [Green Version]
- Ho, A.; Gordon, P.; Rosenthal, E.; Simpson, J.; Miller, O.; Sharland, G. Isolated complete heart block in the fetus. Am. J. Cardiol. 2015, 116, 142–147. [Google Scholar] [CrossRef]
- Brito-Zerón, P.; Izmirly, P.M.; Ramos-Casals, M.; Buyon, J.P.; Khamashta, M.A. The clinical spectrum of autoimmune congenital heart block. Nat. Rev. Rheumatol. 2015, 11, 301–312. [Google Scholar] [CrossRef]
- Panaitescu, A.M.; Nicolaides, K. Maternal autoimmune disorders and fetal defects. In Journal of Maternal-Fetal and Neonatal Medicine; Taylor and Francis Ltd.: London, UK, 2018; Volume 31, pp. 1798–1806. [Google Scholar]
- Jaeggi, E.; Laskin, C.; Hamilton, R.; Silverman, E. The importance of the level of maternal anti-Ro/SSA antibodies as a prognostic marker of the development of cardiac neonatal lupus erythematosus: A prospective study of 186 antibody-exposed fetuses and infants. J. Am. Coll. Cardiol. 2010, 55, 2778–2784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera, T.L.; Izmirly, P.M.; Birnbaum, B.K.; Byrne, P.; Brauth, J.B.; Katholi, M.; Kim, M.Y.; Fischer, J.; Clancy, R.M.; Buyon, J.P. Disease progression in mothers of children enrolled in the Research Registry for Neonatal Lupus. Ann. Rheum. Dis. 2009, 68, 828–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provost, T.T.; Watson, R.; Gammon, W.R.; Radowsky, M.; Harley, J.B.; Reichlin, M. The neonatal lupus syndrome associated with U1RNP (nRNP) antibodies. N. Engl. J. Med. 1987, 316, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Buyon, J.P.; Clancy, R.M.; Friedman, D.M. Cardiac manifestations of neonatal lupus erythematosus: Guidelines to management, integrating clues from the bench and bedside. Nat. Clin. Pract. Rheumatol. 2009, 5, 139–148. [Google Scholar] [CrossRef]
- Cooley, H.M.; Keech, C.L.; Melny, B.J.; Menahem, S.; Morahan, G.; Kay, T.W. Monozygotic twins discordant for congenital complete heart block. Arthritis Rheum. 1997, 40, 381–384. [Google Scholar] [CrossRef]
- Clancy, R.M.; Kapur, R.P.; Molad, Y.; Askanase, A.D.; Buyon, J.P. Immunohistologic evidence supports apoptosis, lgG deposition, and novel macrophage/fibroblast crosstalk in the pathologic cascade leading to congenital heart block. Arthritis Rheum. 2004, 50, 173–182. [Google Scholar] [CrossRef]
- Qu, Y.S.; Lazzerini, P.E.; Capecchi, P.L.; Laghi-Pasini, F.; El Sherif, N.; Boutjdir, M. Autoimmune calcium channelopathies and cardiac electrical abnormalities. Front. Cardiovasc. Med. 2019, 6, 54. [Google Scholar] [CrossRef]
- Izmirly, P.; Saxena, A.; Buyon, J.P. Progress in the pathogenesis and treatment of cardiac manifestations of neonatal lupus. Curr. Opin. Rheumatol. 2017, 29, 467–472. [Google Scholar] [CrossRef] [Green Version]
- Boutjdir, M. Molecular and ionic basis of congenital complete heart block. Trends Cardiovasc. Med. 2000, 10, 114–122. [Google Scholar] [CrossRef]
- Clancy, R.M.; Neufing, P.J.; Zheng, P.; O’Mahony, M.; Nimmerjahn, F.; Gordon, T.P.; Buyon, J.P. Impaired clearance of apoptotic cardiocytes is linked to anti-SSA/Ro and -SSB/La antibodies in the pathogenesis of congenital heart block. J. Clin. Invest. 2006, 116, 2413–2422. [Google Scholar] [CrossRef] [Green Version]
- Miranda-Carús, M.E.; Askanase, A.D.; Clancy, R.M.; Di Donato, F.; Chou, T.M.; Libera, M.R.; Chan, E.K.; Buyon, J.P. Anti-SSA/Ro and Anti-SSB/La Autoantibodies Bind the Surface of Apoptotic Fetal Cardiocytes and Promote Secretion of TNF-α by Macrophages. J. Immunol. 2000, 165, 5345–5351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buyon, J.P.; Clancy, R.M. Autoantibody-associated congenital heart block: TGFh and the road to scar. Autoimmun. Rev. 2004, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Clancy, R.M.; Backer, C.B.; Yin, X.; Kapur, R.P.; Molad, Y.; Buyon, J.P. Cytokine polymorphisms and histologic expression in autopsy studies: Contribution of TNF-α and TGF-β1 to the pathogenesis of autoimmune-associated congenital heart block. J. Immunol. 2003, 171, 3253–3261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clancy, R.M.; Halushka, M.; Rasmussen, S.E.; Lhakhang, T.; Chang, M.; Buyon, J.P. Siglec-1 macrophages and the contribution of IFN to the development of autoimmune congenital heart block. J. Immunol. 2019, 202, 48–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedlund, M.; Thorlacius, G.E.; Ivanchenko, M.; Ottosson, V.; Kyriakidis, N.; Lagnefeldt, L.; Tingström, J.; Sirsjö, A.; Bengtsson, A.A.; Aronsson, E.; et al. Type i IFN system activation in newborns exposed to Ro/SSA and La/SSB autoantibodies in utero. RMD Open 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boutjdir, M.; Chen, L.; Zhang, Z.H.; Tseng, C.E.; El-Sherif, N.; Buyon, J.P. Serum and immunoglobulin G from the mother of a child with congenital heart block induce conduction abnormalities and inhibit L-type calcium channels in a rat heart model. Pediatr. Res. 1998, 44, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Boutjdir, M.; Chen, L.; Zhang, Z.H.; Tseng, C.E.; DiDonato, F.; Rashbaum, W.; Morris, A.; El-Sherif, N.; Buyon, J.P. Arrhythmogenicity of lgG and anti-52-kD SSA/Ro affinity-purified antibodies from mothers of children with congenital heart block. Circ. Res. 1997, 80, 354–362. [Google Scholar] [CrossRef]
- Karnabi, E.; Qu, Y.; Wadgaonkar, R.; Mancarella, S.; Yue, Y.; Chahine, M.; Clancy, R.M.; Buyon, J.P.; Boutjdir, M. Congenital heart block: Identification of autoantibody binding site on the extracellular loop (domain I, S5-S6) of α1D L-type Ca channel. J. Autoimmun. 2010, 34, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Lazzerini, P.E.; Capecchi, P.L.; Laghi-Pasini, F.; Boutjdir, M. Autoimmune channelopathies as a novel mechanism in cardiac arrhythmias. Nat. Rev. Cardiol. 2017, 14, 521–535. [Google Scholar] [CrossRef]
- Jobling, K.; Rajabally, H.; Ng, W.F. Anti-Ro antibodies and complete heart block in adults with Sjögren’s syndrome. Eur. J. Rheumatol. 2018, 5, 194–196. [Google Scholar] [CrossRef]
- Xiao, G.Q.; Hu, K.; Boutjdir, M. Direct inhibition of expressed cardiac L- and T-type calcium channels by IgG from mothers whose children have congenital heart block. Circulation 2001, 103, 1599–1604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambrosi, A.; Wahren-Herlenius, M. Congenital heart block: Evidence for a pathogenic role of maternal autoantibodies. Arthritis Res. Ther. 2012, 14, 208. [Google Scholar] [CrossRef] [Green Version]
- Kan, N.; Silverman, E.D.; Kingdom, J.; Dutil, N.; Laskin, C.; Jaeggi, E. Serial echocardiography for immune-mediated heart disease in the fetus: Results of a risk-based prospective surveillance strategy. Prenat. Diagn. 2017, 37, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Silverman, E.; Jaeggi, E. Non-cardiac manifestations of neonatal lupus erythematosus. In Scandinavian Journal of Immunology; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2010; Volume 72, pp. 223–225. [Google Scholar]
- Neiman, A.R.; Lee, L.A.; Weston, W.L.; Buyon, J.P. Cutaneous manifestations of neonatal lupus without heart block: Characteristics of mothers and children enrolled in a national registry. J. Pediatr. 2000, 137, 674–680. [Google Scholar] [CrossRef]
- Chadha, A.; Jahnke, M. Common neonatal rashes. Pediatr. Ann. 2019, 48, e16–e22. [Google Scholar] [CrossRef] [PubMed]
- Guinovart, R.M.; Vicente, A.; Rovira, C.; Suñol, M.; González-Enseñat, M.A. Facial telangiectasia: An unusual manifestation of neonatal lupus erythematosus. Lupus 2012, 21, 552–555. [Google Scholar] [CrossRef]
- Martin, V.; Lee, L.A.; Askanase, A.D.; Katholi, M.; Buyon, J.P. Long-term followup of children with neonatal lupus and their unaffected siblings. Arthritis Rheum. 2002, 46, 2377–2383. [Google Scholar] [CrossRef]
- Roberts, N.K.; Gelband, H. Arrhythmias in the neonate. In Cardiac Arrhythmias in the Neonate, Infant, and Child; Appleton-Century-Crofts: New York, NY, USA, 1977; p. 533. [Google Scholar]
- Baruteau, A.E.; Pass, R.H.; Thambo, J.B.; Behaghel, A.; Le Pennec, S.; Perdreau, E.; Combes, N.; Liberman, L.; McLeod, C.J. Congenital and childhood atrioventricular blocks: Pathophysiology and contemporary management. Eur. J. Pediatr. 2016, 175, 1235–1248. [Google Scholar] [CrossRef]
- Sekar, R. Hydrops Fetalis. In Complications of Pregnancy; IntechOpen: London, UK, 2019. [Google Scholar]
- Derderian, S.C.; Jeanty, C.; Fleck, S.R.; Cheng, L.S.; Peyvandi, S.; Moon-Grady, A.J.; Farrell, J.; Hirose, S.; Gonzalez, J.; Keller, R.L.; et al. The many faces of hydrops. J. Pediatr. Surg. 2015, 50, 50–54. [Google Scholar] [CrossRef] [Green Version]
- Cuneo, B.F.; Strasburger, J.F.; Niksch, A.; Ovadia, M.; Wakai, R.T. An expanded phenotype of maternal SSASSB antibody-associated fetal cardiac disease. J. Matern. Neonatal Med. 2009, 22, 233–238. [Google Scholar] [CrossRef]
- Cuneo, B.F.; Fruitman, D.; Benson, D.W.; Ngan, B.Y.; Liske, M.R.; Wahren-Herlineus, M.; Ho, S.Y.; Jaeggi, E. Spontaneous rupture of atrioventricular valve tensor apparatus as late manifestation of anti-Ro/SSA antibody-mediated cardiac disease. Am. J. Cardiol. 2011, 107, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Morel, N.; Lévesque, K.; Maltret, A.; Baron, G.; Hamidou, M.; Orquevaux, P.; Piette, J.C.; Barriere, F.; Le Bidois, J.; Fermont, L.; et al. Incidence, risk factors, and mortality of neonatal and late-onset dilated cardiomyopathy associated with cardiac neonatal lupus. Int. J. Cardiol. 2017, 248, 263–269. [Google Scholar] [CrossRef]
- Askanase, A.D.; Friedman, D.M.; Copel, J.; Dische, M.R.; Dubin, A.; Starc, T.J.; Katholi, M.C.; Buyon, J.P. Spectrum and progression of conduction abnormalities in infants born to mothers with anti-SSA/Ro-SSB/La antibodies. Lupus 2002, 11, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Chockalingam, P.; Jaeggi, E.T.; Rammeloo, L.A.; Haak, M.C.; Adama Van Scheltema, P.N.; Breur, J.M.P.J.; Bartelings, M.M.; Clur, S.A.B.; Blom, N.A. Persistent fetal sinus bradycardia associated with maternal anti-SSA/Ro and anti-SSB/La antibodies. J. Rheumatol. 2011, 38, 2682–2685. [Google Scholar] [CrossRef] [PubMed]
- Moak, J.P.; Barron, K.S.; Hougen, T.J.; Wiles, H.B.; Balaji, S.; Sreeram, N.; Cohen, M.H.; Nordenberg, A.; Van Hare, G.F.; Friedman, R.A.; et al. Congenital heart block: Development of late-onset cardiomyopathy, a previously underappreciated sequela. J. Am. Coll. Cardiol. 2001, 37, 238–242. [Google Scholar] [CrossRef] [Green Version]
- Nield, L.E.; Silverman, E.D.; Taylor, G.P.; Smallhorn, J.F.; Mullen, J.B.; Silverman, N.H.; Finley, J.P.; Law, Y.M.; Human, D.G.; Seaward, P.G.; et al. Maternal anti-Ro and anti-La antibody—Associated endocardial fibroelastosis. Circulation 2002, 105, 843–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, L.A.; Sokol, R.J.; Buyon, J.P. Hepatobiliary disease in neonatal lupus: Prevalence and clinical characteristics in cases enrolled in a national registry. Pediatrics 2002, 109, e11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, L.A. Neonatal Lupus: Clinical Features and Management. Pediatr. Drugs 2004, 6, 71–78. [Google Scholar] [CrossRef]
- Olney, R.S.; Ailes, E.C.; Sontag, M.K. Detection of critical congenital heart defects: Review of contributions from prenatal and newborn screening. Semin. Perinatol. 2015, 39, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Donofrio, M.T.; Moon-Grady, A.J.; Hornberger, L.K.; Copel, J.A.; Sklansky, M.S.; Abuhamad, A.; Cuneo, B.F.; Huhta, J.C.; Jonas, R.A.; Krishnan, A.; et al. Diagnosis and treatment of fetal cardiac disease: A scientific statement from the american heart association. Circulation 2014, 129, 2183–2242. [Google Scholar] [CrossRef]
- Glickstein, J.S.; Buyon, J.; Friedman, D. Pulsed Doppler echocardiographic assessment of the fetal PR interval. Am. J. Cardiol. 2000, 86, 236–239. [Google Scholar] [CrossRef]
- Glickstein, J.; Buyon, J.; Kim, M.; Friedman, D.; Abuhamad, A.; Branch, D.W.; Sullivan, A.; Cohen, M.; Copel, J.; Donofrio, M.; et al. The fetal Doppler mechanical PR interval: A validation study. Fetal Diagn. Ther. 2004, 19, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Wojakowski, A.; Izbizky, G.; Carcano, M.E.; Aiello, H.; Marantz, P.; Otaño, L. Fetal Doppler mechanical PR interval: Correlation with fetal heart rate, gestational age and fetal sex. Ultrasound Obstet. Gynecol. 2009, 34, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.M.; Kim, M.Y.; Copel, J.A.; Davis, C.; Phoon, C.K.L.; Glickstein, J.S.; Buyon, J.P. Utility of cardiac monitoring in fetuses at risk for congenital heart block: The PR interval and dexamethasone evaluation (PRIDE) prospective study. Circulation 2008, 117, 485–493. [Google Scholar] [CrossRef] [Green Version]
- Pruetz, J.D.; Miller, J.C.; Loeb, G.E.; Silka, M.J.; Bar-Cohen, Y.; Chmait, R.H. Prenatal diagnosis and management of congenital complete heart block. Birth Defects Res. 2019, 111, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Jaeggi, E.T.; Hamilton, R.M.; Silverman, E.D.; Zamora, S.A.; Hornberger, L.K. Outcome of children with fetal, neonatal or childhood diagnosis of isolated congenital atrioventricular block. J. Am. Coll. Cardiol. 2002, 39, 130–137. [Google Scholar] [CrossRef]
- Izmirly, P.M.; Costedoat-Chalumeau, N.; Pisoni, C.N.; Khamashta, M.A.; Kim, M.Y.; Saxena, A.; Friedman, D.; Llanos, C.; Piette, J.C.; Buyon, J.P. Maternal use of hydroxychloroquine is associated with a reduced risk of recurrent anti-SSA/ro-antibody—Associated cardiac manifestations of neonatal lupus. Circulation 2012, 126, 76–82. [Google Scholar] [CrossRef]
- Izmirly, P.M.; Kim, M.Y.; Llanos, C.; Le, P.U.; Guerra, M.M.; Askanase, A.D.; Salmon, J.E.; Buyon, J.P. Evaluation of the risk of anti-SSA/Ro-SSB/La antibody-associated cardiac manifestations of neonatal lupus in fetuses of mothers with systemic lupus erythematosus exposed to hydroxychloroquine. Ann. Rheum. Dis. 2010, 69, 1827–1830. [Google Scholar] [CrossRef]
- Skorpen, C.G.; Hoeltzenbein, M.; Tincani, A.; Fischer-Betz, R.; Elefant, E.; Chambers, C.; Da Silva, J.; Nelson-Piercy, C.; Cetin, I.; Costedoat-Chalumeau, N.; et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann. Rheum. Dis. 2016, 75, 795–810. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, C.; Avina-Zubieta, A.; Rai, S.K.; Carruthers, E.; De Vera, M.A. Disease-modifying anti-rheumatic drug use in pregnant women with rheumatic diseases: A systematic review of the risk of congenital malformations. Clin. Exp. Rheumatol. 2016, 34, 172–183. [Google Scholar]
- Jaeggi, E.T.; Silverman, E.D.; Laskin, C.; Kingdom, J.; Golding, F.; Weber, R. Prolongation of the atrioventricular conduction in fetuses exposed to maternal Anti-Ro/SSA and Anti-La/SSB antibodies did not predict progressive heart block: A prospective observational study on the effects of maternal antibodies on 165 fetuses. J. Am. Coll. Cardiol. 2011, 57, 1487–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clowse, M.E.; Eudy, A.M.; Kiernan, E.; Williams, M.R.; Bermas, B.; Chakravarty, E.; Sammaritano, L.R.; Chambers, C.D.; Buyon, J. The prevention, screening and treatment ofcongenital heart block from neonatal lupus:a survey of provider practices. Rheumatology 2018, 57 (Suppl. S5), v9–v17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rein, A.J.J.T.; Mevorach, D.; Perles, Z.; Gavri, S.; Nadjari, M.; Nir, A.; Elchalal, U. Early diagnosis and treatment of atrioventricular block in the fetus exposed to maternal anti-SSA/Ro-SSB/La antibodies a prospective, observational, fetal kinetocardiogram-based study. Circulation 2009, 119, 1867–1872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mevorach, D.; Elchalal, U.; Rein, A.J.J.T. Prevention of complete heart block in children of mothers with anti-SSA/Ro and anti-SSB/La autoantibodies: Detection and treatment of first-degree atrioventricular block. Curr. Opin. Rheumatol. 2009, 21, 478–482. [Google Scholar] [CrossRef]
- Ciardulli, A.; D’Antonio, F.; Magro-Malosso, E.R.; Manzoli, L.; Anisman, P.; Saccone, G.; Berghella, V. Maternal steroid therapy for fetuses with second-degree immune-mediated congenital atrioventricular block: A systematic review and meta-analysis. Acta Obstet. Gynecol. Scand. 2018, 97, 787–794. [Google Scholar] [CrossRef] [Green Version]
- Friedman, D.M.; Kim, M.Y.; Copel, J.A.; Llanos, C.; Davis, C.; Buyon, J.P. Prospective evaluation of fetuses with autoimmune-associated congenital heart block followed in the PR Interval and Dexamethasone Evaluation (PRIDE) Study. Am. J. Cardiol. 2009, 103, 1102–1106. [Google Scholar] [CrossRef] [Green Version]
- Cuneo, B.F.; Ambrose, S.E.; Tworetzky, W. Detection and successful treatment of emergent anti-SSA–mediated fetal atrioventricular block. Am. J. Obstet. Gynecol. 2016, 215, 527–528. [Google Scholar] [CrossRef] [Green Version]
- Cuneo, B.F.; Moon-Grady, A.J.; Sonesson, S.E.; Levasseur, S.; Hornberger, L.; Donofrio, M.T.; Krishnan, A.; Szwast, A.; Howley, L.; Benson, D.W.; et al. Heart sounds at home: Feasibility of an ambulatory fetal heart rhythm surveillance program for anti-SSA-positive pregnancies. J. Perinatol. 2017, 37, 226–230. [Google Scholar] [CrossRef]
- Doti, P.I.; Escoda, O.; Cesar-Díaz, S.; Palasti, S.; Teixidó, I.; Sarquella-Brugada, G.; Gómez, O.; Martínez, J.M.; Espinosa, G. Congenital heart block related to maternal autoantibodies: Descriptive analysis of a series of 18 cases from a single center. Clin. Rheumatol. 2016, 35, 351–356. [Google Scholar] [CrossRef]
- Levesque, K.; Morel, N.; Maltret, A.; Baron, G.; Masseau, A.; Orquevaux, P.; Piette, J.C.; Barriere, F.; Le Bidois, J.; Fermont, L.; et al. Description of 214 cases of autoimmune congenital heart block: Results of the French neonatal lupus syndrome. Autoimmun. Rev. 2015, 14, 1154–1160. [Google Scholar] [CrossRef]
- Izmirly, P.M.; Saxena, A.; Sahl, S.K.; Shah, U.; Friedman, D.M.; Kim, M.Y.; Buyon, J.P. Assessment of fluorinated steroids to avert progression and mortality in anti-SSA/Ro-associated cardiac injury limited to the fetal conduction system. Ann. Rheum. Dis. 2016, 75, 1161–1165. [Google Scholar] [CrossRef]
- Ruffatti, A.; Cerutti, A.; Favaro, M.; Del Ross, T.; Calligaro, A.; Hoxha, A.; Marson, P.; Leoni, L.; Milanesi, O. Plasmapheresis, intravenous immunoglobulins and bethametasone-a combined protocol to treat autoimmune congenital heart block: A prospective cohort study. Clin. Exp. Rheumatol. 2016, 34, 706–713. [Google Scholar]
- Tonello, M.; Ruffatti, A.; Marson, P.; Tison, T.; Marozio, L.; Hoxha, A.; De Silvestro, G.; Punzi, L. Plasma exchange effectively removes 52- and 60-kDa anti-RO/SSA and anti-La/SSB antibodies in pregnant women with congenital heart block. Transfusion 2015, 55, 1782–1786. [Google Scholar] [CrossRef] [PubMed]
- Jaeggi, E.T.; Fouron, J.C.; Silverman, E.D.; Ryan, G.; Smallhorn, J.; Hornberger, L.K. Transplacental fetal treatment improves the outcome of prenatally diagnosed complete atrioventricular block without structural heart disease. Circulation 2004, 110, 1542–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, B.V.; Ettedgui, J.A.; Sherman, F.S. Use of terbutaline in the treatment of complete heart block in the fetus. Cardiol. Young 2001, 11, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Eghtesady, P.; Michelfelder, E.C.; Knilans, T.K.; Witte, D.P.; Manning, P.B.; Crombleholme, T.M. Fetal surgical management of congenital heart block in a hydropic fetus: Lessons learned from a clinical experience. J. Thorac. Cardiovasc. Surg. 2011, 141, 835–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusumoto, F.M.; Schoenfeld, M.H.; Barrett, C.; Edgerton, J.R.; Ellenbogen, K.A.; Gold, M.R.; Goldschlager, N.F.; Hamilton, R.M.; Joglar, J.A.; Kim, R.J.; et al. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 74, 932–987. [Google Scholar] [CrossRef] [PubMed]
- Stanner, C.; Horndasch, M.; Vitanova, K.; Strbad, M.; Ono, M.; Hessling, G.; Lange, R.; Cleuziou, J. Neonates and infants requiring life-long cardiac pacing: How reliable are epicardial leads through childhood? Int. J. Cardiol. 2019, 297, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Ergün, S.; Kafalı, H.C.; Genç, S.B.; Yildiz, O.; Güneş, M.; Onan, İ.S.; Ergül, Y.; Güzeltaş, A.; Haydin, S. Epicardial Pacemaker in Neonates and Infants: Is There a Relationship Between Patient Size, Device Size, and Wound Complicatıon? Pediatr. Cardiol. 2020, 1, 1–9. [Google Scholar] [CrossRef]
- Clancy, R.M.; Marion, M.C.; Kaufman, K.M.; Ramos, P.S.; Adler, A.; Harley, J.B.; Langefeld, C.D.; Buyon, J.P. Identification of candidate loci at 6p21 and 21q22 in a genome-wide association study of cardiac manifestations of neonatal lupus. Arthritis Rheum. 2010, 62, 3415–3424. [Google Scholar] [CrossRef] [Green Version]
- Manolis, A.A.; Manolis, T.A.; Melita, H.; Manolis, A.S. Congenital heart block: Pace earlier (Childhood) than later (Adulthood). In Trends in Cardiovascular Medicine; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Alvarez, D.; Briassouli, P.; Clancy, R.M.; Zavadil, J.; Reed, J.H.; Abellar, R.G.; Halushka, M.; Fox-Talbot, K.; Barrat, F.J.; Buyon, J.P. A novel role of endothelin-1 in linking toll-like receptor 7-mediated inflammation to fibrosis in congenital heart block. J. Biol. Chem. 2011, 286, 30444–30454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jousse-Joulin, S.; D’Agostino, M.A.; Nicolas, C.; Naredo, E.; Ohrndorf, S.; Backhaus, M.; Tamborrini, G.; Chary-Valckenaere, I.; Terslev, L.; Iagnocco, A.; et al. Video clip assessment of a salivary gland ultrasound scoring system in Sjögren’s syndrome using consensual definitions: An OMERACT ultrasound working group reliability exercise. Ann. Rheum. Dis. 2019, 78, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Buyon, J. Preventive Approach to Congenital Heart Block with Hydroxychloroquine-Study Results-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/results/NCT01379573?view=results (accessed on 19 May 2020).
- Izmirly, P.; Kim, M.; Costedoat-Chalumeau, N.; Friedman, D.; Saxena, A.; Copel, J.; Cohen, R.; Masson, M.; Middleton, T.; Robins, K.; et al. The prospective open label Preventive Approach to Congenital Heart Block with Hydroxychloroquine (PATCH) Study Demonstrates a Reduction in the recurrence rate of advanced block—Acr meeting abstracts. Arthritis Rheumatol. 2019, 71 (Suppl. S10), 111. [Google Scholar]
- Andelfinger, G.; Fouron, J.C.; Sonesson, S.E.; Proulx, F. Reference values for time intervals between atrial and ventricular contractions of the fetal heart measured by two Doppler techniques. Am. J. Cardiol. 2001, 88, 1433–1436. [Google Scholar] [CrossRef]
- Sonesson, S.E.; Salomonsson, S.; Jacobsson, L.A.; Bremme, K.; Wahren-Herlenius, M. Signs of first-degree heart block occur in one-third of fetuses of pregnant women with anti-SSA/Ro 52-kd antibodies. Arthritis Rheum. 2004, 50, 1253–1261. [Google Scholar] [CrossRef]
- Carvalho, J.S. Fetal dysrhythmias. In Best Practice and Research: Clinical Obstetrics and Gynaecology; Bailliere Tindall Ltd.: London, UK, 2019; Volume 58, pp. 28–41. [Google Scholar]
- Eliasson, H.; Wahren-Herlenius, M.; Sonesson, S.E. Mechanisms in fetal bradyarrhythmia: 65 cases in a single center analyzed by Doppler flow echocardiographic techniques. Ultrasound Obstet. Gynecol. 2011, 37, 172–178. [Google Scholar] [CrossRef]
- Sonesson, S.-E. Diagnosing Foetal Atrioventricular Heart Blocks. Scand. J. Immunol. 2010, 72, 205–212. [Google Scholar] [CrossRef]
- von Steinburg, S.P.; Boulesteix, A.L.; Lederer, C.; Grunow, S.; Schiermeier, S.; Hatzmann, W.; Schneider, K.T.M.; Daumer, M. What is the “normal” fetal heart rate? PeerJ 2013, 1, e82. [Google Scholar] [CrossRef] [Green Version]
- Jaeggi, E.T.; Nii, M. Fetal brady- and tachyarrhythmias: New and accepted diagnostic and treatment methods. Semin. Fetal Neonatal Med. 2005, 10, 504–514. [Google Scholar] [CrossRef]
- Cimaz, R.; Stramba-Badiale, M.; Brucato, A.; Catelli, L.; Panzeri, P.; Meroni, P.L. QT interval prolongation in asymptomatic anti-SSA/Ro-positive infants without congenital heart block. Arthritis Rheum. 2000, 43, 1049–1053. [Google Scholar] [CrossRef]
- Capone, C.; Buyon, J.P.; Friedman, D.M.; Frishman, W.H. Cardiac Manifestations of Neonatal Lupus. Cardiol. Rev. 2012, 20, 72–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karpawich, P.P.; Rabah, R.; Haas, J.E. Altered cardiac histology following apical right ventricular pacing in patients with congenital atrioventricular block. PACE Pacing Clin. Electrophysiol. 1999, 22, 1372–1377. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Friedman, R.A.; Eidem, B.W.; Cannon, B.C.; Arora, G.; Smith, E.O.B.; Fenrich, A.L.; Kertesz, N.J. Ventricular function and long-term pacing in children with congenital complete atrioventricular block. J. Cardiovasc. Electrophysiol. 2007, 18, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Udink Ten Cate, F.E.A.; Breur, J.M.P.J.; Cohen, M.I.; Boramanand, N.; Kapusta, L.; Crosson, J.E.; Brenner, J.I.; Lubbers, L.J.; Friedman, A.H.; Vetter, V.L.; et al. Dilated cardiomyopathy in isolated congenital complete atrioventricular block: Early and long-term risk in children. J. Am. Coll. Cardiol. 2001, 37, 1129–1134. [Google Scholar] [CrossRef] [Green Version]
- Silvetti, M.S.; Drago, F.; Ravà, L. Determinants of early dilated cardiomyopathy in neonates with congenital complete atrioventricular block. Europace 2010, 12, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Mofors, J.; Eliasson, H.; Ambrosi, A.; Salomonsson, S.; Skog, A.; Fored, M.; Ekbom, A.; Bergman, G.; Sonesson, S.E.; Wahren-Herlenius, M. Comorbidity and long-term outcome in patients with congenital heart block and their siblings exposed to Ro/SSA autoantibodies in utero. Ann. Rheum. Dis. 2019, 78, 696–703. [Google Scholar] [CrossRef] [Green Version]
- Cimaz, R.; Meroni, P.L.; Brucato, A.; Fesstovà, V.; Panzeri, P.; Goulene, K.; Stramba-Badiale, M. Concomitant disappearance of electrocardiographic abnormalities and of acquired maternal autoantibodies during the first year of life in infants who had QT interval prolongation and anti-SSA/Ro positivity without congenital heart block at birth. Arthritis Rheum. 2003, 48, 266–268. [Google Scholar] [CrossRef]
- Tufan, A.N.; Sag, S.; Oksuz, M.F.; Ermurat, S.; Coskun, B.N.; Gullulu, M.; Budak, F.; Baran, I.; Pehlivan, Y.; Dalkilic, E. Prolonged Tpeak–Tend interval in anti-Ro52 antibody-positive connective tissue diseases. Rheumatol. Int. 2017, 37, 67–73. [Google Scholar] [CrossRef]
- Bourré-Tessier, J.; Clarke, A.E.; Huynh, T.; Bernatsky, S.; Joseph, L.; Belisle, P.; Pineau, C.A. Prolonged corrected QT interval in anti-Ro/SSA-positive adults with systemic lupus erythematosus. Arthritis Care Res. 2011, 63, 1031–1037. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Capecchi, P.L.; Acampa, M.; Morozzi, G.; Bellisai, F.; Bacarelli, M.R.; Dragoni, S.; Fineschi, I.; Simpatico, A.; Galeazzi, M.; et al. Anti-Ro/SSA-associated corrected QT interval prolongation in adults: The role of antibody level and specificity. Arthritis Care Res. 2011, 63, 1463–1470. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Capecchi, P.L.; Laghi-Pasini, F. Long QT syndrome: An emerging role for inflammation and immunity. Front. Cardiovasc. Med. 2015, 2, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazzerini, P.E.; Capecchi, P.L.; El-Sherif, N.; Laghi-Pasini, F.; Boutjdir, M. Emerging arrhythmic risk of autoimmune and inflammatory cardiac channelopathies. J. Am. Heart Assoc. 2018, 7, e010595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duke, C.; Stuart, G.; Simpson, J.M. Ventricular tachycardia secondary to prolongation of the QT interval in a fetus with autoimmune mediated congenital complete heart block. Cardiol. Young 2005, 15, 319–321. [Google Scholar] [CrossRef]
- Nakamura, K.; Katayama, Y.; Kusano, K.F.; Haraoka, K.; Tani, Y.; Nagase, S.; Morita, H.; Miura, D.; Fujimoto, Y.; Furukawa, T.; et al. Anti-KCNH2 antibody-induced long QT syndrome. Novel acquired form of long QT syndrome. J. Am. Coll. Cardiol. 2007, 50, 1808–1809. [Google Scholar] [CrossRef] [Green Version]
- Mancarella, S.; Yue, Y.; Karnabi, E.; Qu, Y.; El-Sherif, N.; Boutjdir, M. Impaired Ca2+ homeostasis is associated with atrial fibrillation in the α1D L-type Ca2+ channel KO mouse. Am. J. Physiol. Hear. Circ. Physiol. 2008, 295, H2017–H2024. [Google Scholar] [CrossRef] [Green Version]
- Bergman, G.; Eliasson, H.; Mohlkert, L.-A.; Wahren-Herlenius, M.; Sonesson, S.-E. Progression to first-degree heart block in preschool children exposed in utero to maternal anti-SSA/Ro52 autoantibodies. Acta Paediatr. 2012, 101, 488–493. [Google Scholar] [CrossRef] [Green Version]
- Lazzerini, P.E.; Brucato, A.; Capecchi, P.L.; Baldi, L.; Bacarelli, M.R.; Nucci, C.; Moscadelli, V.; Morozzi, G.; Boutjdir, M.; Laghi-Pasini, F. Isolated atrioventricular block of unknown origin in the adult and autoimmunity: Diagnostic and therapeutic considerations exemplified by 3 anti-Ro/SSA-associated cases. Hear. Case Rep. 2015, 1, 293–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witczak, B.N.; Hetlevik, S.O.; Sanner, H.; Barth, Z.; Schwartz, T.; Flatø, B.; Lilleby, V.; Sjaastad, I. Effect on cardiac function of longstanding juvenile-onset mixed connective tissue disease: A controlled study. J. Rheumatol. 2019, 46, 739–747. [Google Scholar] [CrossRef]
- Leone, P.; Cicco, S.; Prete, M.; Solimando, A.G.; Susca, N.; Crudele, L.; Buonavoglia, A.; Colonna, P.; Dammacco, F.; Vacca, A.; et al. Early echocardiographic detection of left ventricular diastolic dysfunction in patients with systemic lupus erythematosus asymptomatic for cardiovascular disease. Clin. Exp. Med. 2020, 20, 11–19. [Google Scholar] [CrossRef]
- Chang, J.C.; White, B.R.; Elias, M.D.; Xiao, R.; Knight, A.M.; Weiss, P.F.; Mercer-Rosa, L. Echocardiographic Assessment of Diastolic Function in Children with Incident Systemic Lupus Erythematosus. Pediatr. Cardiol. 2019, 40, 1017–1025. [Google Scholar] [CrossRef]
- Pieske, B.; Tschö Pe, C.; De Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA-PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) Heart failure/cardiomyopathy. Eur. Heart J. 2019, 40, 3297–3317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, K.S.; Xu, H.; Matsouaka, R.A.; Bhatt, D.L.; Heidenreich, P.A.; Hernandez, A.F.; Devore, A.D.; Yancy, C.W.; Fonarow, G.C. Heart failure with preserved, borderline, and reduced ejection fraction 5-year outcomes. J. Am. Coll. Cardiol. 2017, 70, 2476–2486. [Google Scholar] [CrossRef] [PubMed]







| Patients with symptoms specific to Sjögren’s syndrome: xerostomia, xerophthalmia, salivary gland enlargement [14] |
| Women with Sjögren’s syndrome or systemic lupus erythematosus who intend to become pregnant [13] |
| Patients with symptoms suggesting the diagnosis of systemic lupus erythematosus, but with a negative indirect immunofluorescence test for antinuclear antibodies [13] |
| Patients with rheumatoid arthritis or juvenile idiopathic arthritis [24] |
| Women with a history of giving birth to a child with congenital heart block or neonatal lupus [24] |
| Antinuclear antibody (ANA)-positive women who are planning to become pregnant |
| Congenital Heart Block AND | Class of Recommendation |
|---|---|
| Symptomatic bradycardia or low cardiac output | I |
| Wide QRS escape rhythm, complex ventricular ectopy or ventricular dysfunction | I |
| Infants with normal anatomy and a ventricular rate less than 55 beats/min | I |
| Infants with other structural congenital heart disease and a ventricular rate less than 70 beats/min | I |
| Asymptomatic adults with congenital CHB | IIa |
| Children beyond the first year of life with an average heart rate less than 50 beats per minute or abrupt pauses two to three times the basic R–R cycle length | IIa |
| Block Degree | US Diagnosis | Obtained Data | FHR Limits [75,113,114,115] | |
|---|---|---|---|---|
| 1st degree | Spectral PW Doppler at the level of mitral inflow and Aortic outlet | PR > 150 ms [79] | Normal heart rate 120–160 bpm [116] | |
| 2nd degree | Type 1 | M-mode; spectral PW | Progressive PR prolongation, until an atrial wave is blocked | Irregular rhythm Normal heart rate |
| 2:1 block | Every other atrial beat is blocked | Regular rhythm 60–80 bpm | ||
| Type 2 | Sudden block of an atrial wave | Regular rhythm Normal heart rate | ||
| 3rd degree 3 | M-mode | Complete A–V dissociation | <60 bpm | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, M.R.; Dudu, A.; Jurcut, C.; Ciobanu, A.M.; Zagrean, A.-M.; Panaitescu, A.M. A Broader Perspective on Anti-Ro Antibodies and Their Fetal Consequences—A Case Report and Literature Review. Diagnostics 2020, 10, 478. https://doi.org/10.3390/diagnostics10070478
Popescu MR, Dudu A, Jurcut C, Ciobanu AM, Zagrean A-M, Panaitescu AM. A Broader Perspective on Anti-Ro Antibodies and Their Fetal Consequences—A Case Report and Literature Review. Diagnostics. 2020; 10(7):478. https://doi.org/10.3390/diagnostics10070478
Chicago/Turabian StylePopescu, Mihaela Roxana, Andreea Dudu, Ciprian Jurcut, Anca Marina Ciobanu, Ana-Maria Zagrean, and Anca Maria Panaitescu. 2020. "A Broader Perspective on Anti-Ro Antibodies and Their Fetal Consequences—A Case Report and Literature Review" Diagnostics 10, no. 7: 478. https://doi.org/10.3390/diagnostics10070478
APA StylePopescu, M. R., Dudu, A., Jurcut, C., Ciobanu, A. M., Zagrean, A. -M., & Panaitescu, A. M. (2020). A Broader Perspective on Anti-Ro Antibodies and Their Fetal Consequences—A Case Report and Literature Review. Diagnostics, 10(7), 478. https://doi.org/10.3390/diagnostics10070478