A Narrative Review of the Classical and Modern Diagnostic Methods of the No-Reflow Phenomenon
Abstract
:1. Introduction
- a NR phenomenon identified by angiography, expressing the degree of TIMI and MBG flow;
- a myocardial NR that may occur in patients who have obtained excellent resumption of intracoronary flow, but in whom the quality of reperfusion in the microcirculation is poor [4].
2. Methods
3. Pathogenesis of the No-Reflow Phenomenon
3.1. Pre-Existing Lesions of the Microcirculation
3.2. Distal Microembolization
3.3. Ischemic Myocardial Injury
3.4. Myocyte Reperfusion Injury
3.5. Individual Susceptibility
4. Predictors of the No-Reflow Phenomenon
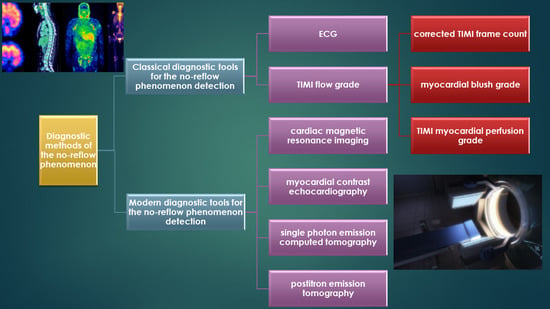
5. Diagnostic Methods of the No-Reflow Phenomenon
5.1. Classical Diagnostic Tools for the No-Reflow Phenomenon Detection
5.1.1. ECG
5.1.2. Coronary Angiography
TIMI Flow Grade
CTFC
MBG
TMPG
5.2. Modern Diagnostic Tools for No-Reflow Phenomenon Detection
5.2.1. CMRI
5.2.2. MCE
5.2.3. SPECT
5.2.4. PET
5.3. Classical Versus Modern Diagnostics Methods of the No-Reflow Phenomenon
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ito, H. No-reflow phenomenon and prognosis in patients with acute myocardial infarction. Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Ndrepepa, G.; Tiroch, K.; Fusaro, M.; Keta, D.; Seyfarth, M.; Byrne, R.A.; Pache, J.; Alger, P.; Mehilli, J.; Schömig, A.; et al. 5-year prognostic value of no-reflow phenomenon after percutaneous coronary intervention in patients with acute myocardial infarction. J. Am. Coll. Cardiol. 2010, 55, 2383–2389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantea-Roșan, L.R.; Pantea, V.A.; Bungau, S.; Tit, D.M.; Behl, T.; Vesa, C.M.; Bustea, C.; Moleriu, R.D.; Rus, M.; Popescu, M.I.; et al. No-reflow after PPCI—A predictor of short-term outcomes in STEMI patients. J. Clin. Med. 2020, 9, 2956. [Google Scholar] [CrossRef] [PubMed]
- Ramjane, K.; Lei, H.; Jin, C. The diagnosis and treatment of the no-reflow phenomenon in patients with myocardial infarction undergoing percutaneous coronary intervention. Exp. Clin. Cardiol. 2008, 13, 121–128. [Google Scholar]
- Harrison, R.W.; Aggarwal, A.; Ou, F.S.; Klein, L.W.; Rumsfeld, J.S.; Roe, M.T.; Wang, T.Y. Incidence and Outcomes of no-reflow phenomenon during percutaneous coronary intervention among patients with acute myocardial infarction. Am. J. Cardiol. 2013, 111, 178–184. [Google Scholar] [CrossRef]
- Barletta, G.; Bene, M.R. Del Myocardial perfusion echocardiography and coronary microvascular dysfunction. World J. Cardiol. 2015, 7, 861–874. [Google Scholar] [CrossRef]
- Allencherril, J.; Jneid, H.; Atar, D.; Alam, M.; Levine, G.; Kloner, R.A.; Birnbaum, Y. Pathophysiology, Diagnosis, and management of the no-reflow phenomenon. Cardiovasc. Drugs Ther. 2019, 33, 589–597. [Google Scholar] [CrossRef]
- Kaur, G.; Baghdasaryan, P.; Natarajan, B.; Sethi, P.; Mukherjee, A.; Varadarajan, P.; Pai, R.G. Pathophysiology, diagnosis, and management of coronary no-reflow phenomenon. Int. J. Angiol. 2021, 30, 15–21. [Google Scholar] [CrossRef]
- Namazi, M.; Mahmoudi, E.; Safi, M.; Jenab, Y.; Va-Kili, H.; Saadat, H.; Parsa, S.A.; Khaheshi, I.; Talasaz, A.H.; Hosseini, S.H.; et al. The No-reflow phenomenon: Is it predictable by demographic factors and routine laboratory data? Acta Biomed. 2021, 92, e2021297. [Google Scholar] [CrossRef]
- Gheorghe, G.; Toth, P.P.; Bungau, S.; Behl, T.; Ilie, M.; Stoian, A.P.; Bratu, O.G.; Bacalbasa, N.; Rus, M.; Diaconu, C.C. Cardiovascular risk and statin therapy considerations in women. Diagnostics 2020, 10, 483. [Google Scholar] [CrossRef]
- Porto, I.; Ashar, V.; Mitchell, A. Pharmacological management of no reflow during percutaneous coronary intervention. Curr. Vasc. Pharmacol. 2006, 4, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Bungau, S.; Kumar, K.; Zengin, G.; Khan, F.; Kumar, A.; Kaur, R.; Venkatachalam, T.; Tit, D.M.; Vesa, C.M.; et al. Pleotropic effects of polyphenols in cardiovascular system. Biomed. Pharmacother. 2020, 130, 110714. [Google Scholar] [CrossRef] [PubMed]
- Sabir, F.; Barani, M.; Mukhtar, M.; Rahdar, A.; Cucchiarini, M.; Zafar, M.N.; Behl, T.; Bungau, S. Nanodiagnosis and nanotreatment of cardiovascular diseases: An overview. Chemosensors 2021, 9, 67. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 105906. [Google Scholar] [CrossRef]
- Mandurino-Mirizzi, A.; Spolverini, M.; Attanasio, A.; Crimi, G. Management of no-reflow: Still an unsolved problem? J. Phlebol. Lymphology 2020, 13, 1–7. [Google Scholar]
- Ito, H.; Maruyama, A.; Iwakura, K.; Takiuchi, S.; Masuyama, T.; Hori, M.; Higashino, Y.; Fujii, K.; Minamino, T. Clinical implications of the ‘no reflow’ phenomenon. Circulation 1996, 93, 223–228. [Google Scholar] [CrossRef]
- Yang, L.; Cong, H.; Lu, Y.; Chen, X.; Liu, Y. Prediction of no-reflow phenomenon in patients treated with primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Medicine 2020, 99, e20152. [Google Scholar] [CrossRef]
- Cenko, E.; Ricci, B.; Kedev, S.; Kalpak, O.; Câlmâc, L.; Vasiljevic, Z.; Knežević, B.; Dilic, M.; Miličić, D.; Manfrini, O.; et al. The no-reflow phenomenon in the young and in the elderly. Int. J. Cardiol. 2016, 222, 1122–1128. [Google Scholar] [CrossRef]
- Li, H.; Fu, D.G.; Liu, F.Y.; Zhou, H.; Li, X.M. Evaluation of related factors, prediction and treatment drugs of no-reflow phenomenon in patients with acute ST-segment elevation myocardial infarction after direct PCI. Exp. Ther. Med. 2018, 15, 3940–3946. [Google Scholar] [CrossRef]
- Morishima, I.; Sone, T.; Mokuno, S.; Taga, S.; Shimauchi, A.; Oki, Y.; Kondo, J.; Tsuboi, H.; Sassa, H. Clinical significance of no-reflow phenomenon observed on angiography after successful treatment of acute myocardial infarction with percutaneous transluminal coronary angioplasty. Am. Heart J. 1995, 130, 239–243. [Google Scholar] [CrossRef]
- Rossington, J.A.; Sol, E.; Masoura, K.; Aznaouridis, K.; Chelliah, R.; Cunnington, M.; Davison, B.; John, J.; Oliver, R.; Hoye, A. No-reflow phenomenon and comparison to the normal-flow population postprimary percutaneous coronary intervention for ST elevation myocardial infarction: Case-control study (NORM PPCI). Open Heart 2020, 7, e001215. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Liu, M.; Wu, C.; Zhang, Q.; Lu, L.; Wang, Z. Risk factors for no-reflow phenomenon after percutaneous coronary intervention in patients with acute coronary syndrome. Rev. Investig. Clin. 2017, 69, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Rezkalla, S.H.; Dharmashankar, K.C.; Abdalrahman, I.B.; Kloner, R.A. No-reflow phenomenon following percutaneous coronary intervention for acute myocardial infarction: Incidence, outcome, and effect of pharmacologic therapy. J. Interv. Cardiol. 2010, 23, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.H.; Taha, N.M.; Baraka, K.; Ashraf, M.; Shehata, S. Clinical and procedural predictors of suboptimal myocardial reperfusion in primary percutaneous coronary intervention. IJC Heart Vasc. 2019, 23, 100357. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.C.; Zerhouni, E.A.; Judd, R.M.; Lugo-Olivieri, C.H.; Barouch, L.A.; Schulman, S.P.; Blumenthal, R.S.; Lima, J.A.C. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation 1998, 97, 765–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oikonomou, E.; Mourouzis, K.; Vogiatzi, G.; Siasos, G.; Deftereos, S.; Papaioannou, S.; Latsios, G.; Tsalamandris, S.; Tousoulis, D. Coronary microcirculation and the no-reflow phenomenon. Curr. Pharm. Des. 2018, 24, 2934–2942. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, M.M. No reflow phenomenon in percutaneous coronary interventions in ST-segment elevation myocardial infarction. Indian Heart J. 2016, 68, 539–551. [Google Scholar] [CrossRef] [Green Version]
- Jaffe, R.; Charron, T.; Puley, G.; Dick, A.; Strauss, B.H. Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation 2008, 117, 3152–3156. [Google Scholar] [CrossRef] [Green Version]
- Katayama, Y.; Taruya, A.; Kashiwagi, M.; Ozaki, Y.; Shiono, Y.; Tanimoto, T.; Yoshikawa, T.; Kondo, T.; Tanaka, A. No-reflow phenomenon and in vivo cholesterol crystals combined with lipid core in acute myocardial infarction. Int. J. Cardiol. Heart Vasc. 2022, 38, 100953. [Google Scholar] [CrossRef]
- Niccoli, G.; Scalone, G.; Lerman, A.; Crea, F. Coronary microvascular obstruction in acute myocardial infarction. Eur. Heart J. 2016, 37, 1024–1033. [Google Scholar] [CrossRef] [Green Version]
- Patel, K.K.; Spertus, J.A.; Chan, P.S.; Sperry, B.W.; Thompson, R.C.; Al Badarin, F.; Kennedy, K.F.; Case, J.A.; Courter, S.; Saeed, I.M.; et al. Extent of myocardial ischemia on positron emission tomography and survival benefit with early revascularization. J. Am. Coll. Cardiol. 2019, 74, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Minicucci, M.F.; Azevedo, P.S.; Polegato, B.F.; Paiva, S.A.R.; Zornoff, L.A.M. Heart failure after myocardial infarction: Clinical implications and treatment. Clin. Cardiol. 2011, 34, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Yellon, D.M.; Hausenloy, D.J. Myocardial reperfusion injury. N. Engl. J. Med. 2007, 357, 1121–1135. [Google Scholar] [CrossRef] [PubMed]
- Bekkers, S.C.A.M.; Yazdani, S.K.; Virmani, R.; Waltenberger, J. Microvascular obstruction: Underlying pathophysiology and clinical diagnosis. J. Am. Coll. Cardiol. 2010, 55, 1649–1660. [Google Scholar] [CrossRef] [Green Version]
- Vrints, C.J.M. Pathophysiology of the no-reflow phenomenon. Acute Card. Care 2009, 11, 69–76. [Google Scholar] [CrossRef]
- Fajar, J.K.; Heriansyah, T.; Rohman, M.S. The predictors of no reflow phenomenon after percutaneous coronary intervention in patients with ST elevation myocardial infarction: A meta-analysis. Indian Heart J. 2018, 70, S406–S418. [Google Scholar] [CrossRef]
- Shankar, S.S.; Steinberg, H.O. Obesity and endothelial dysfunction. Semin. Vasc. Med. 2005, 5, 56–64. [Google Scholar] [CrossRef]
- Okutsu, M.; Mitomo, S.; Nakamura, S.; Nakamura, S. Interventional cardiology are the high-risk plaques of no-reflow phenomenon equivalent to vulnerable plaques? Interv. Cardiol. 2021, 13, 64–68. [Google Scholar]
- Hong, Y.J.; Jeong, M.H.; Choi, Y.H.; Ko, J.S.; Lee, M.G.; Kang, W.Y.; Lee, S.E.; Kim, S.H.; Park, K.H.; Sim, D.S.; et al. Impact of plaque components on no-reflow phenomenon after stent deployment in patients with acute coronary syndrome: A virtual histology-intravascular ultrasound analysis. Eur. Heart J. 2011, 32, 2059–2066. [Google Scholar] [CrossRef]
- Obaid, D.R.; Calvert, P.A.; Gopalan, D.; Parker, R.A.; Hoole, S.P.; West, N.E.J.; Goddard, M.; Rudd, J.H.F.; Bennett, M.R. Atherosclerotic plaque composition and classification identified by coronary computed tomography: Assessment of computed tomography-generated plaque maps compared with virtual histology intravascular ultrasound and histology. Circ. Cardiovasc. Imaging 2013, 6, 655–664. [Google Scholar] [CrossRef]
- Shioi, A.; Ikari, Y. Plaque calcification during atherosclerosis progression and regression. J. Atheroscler. Thromb. 2018, 25, 294–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, I.; den Ruijter, H.; Nahrendorf, M.; Pasterkamp, G. Detecting the vulnerable plaque in patients. J. Intern. Med. 2015, 278, 520–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katakami, N. Mechanism of development of atherosclerosis and cardiovascular disease in diabetes mellitus. J. Atheroscler. Thromb. 2018, 25, 27–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, C.S.; Golden, S.H.; Anderson, C.; Bray, G.A.; Burke, L.E.; De Boer, I.H.; Deedwania, P.; Eckel, R.H.; Ershow, A.G.; Fradkin, J.; et al. Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: A scientific statement from the American heart association and the American diabetes association. Diabetes Care 2015, 38, 1777–1803. [Google Scholar] [CrossRef] [Green Version]
- Bolad, I.; Delafontaine, P. Endothelial dysfunction: Its role in hypertensive coronary disease. Curr. Opin. Cardiol. 2005, 20, 270–274. [Google Scholar] [CrossRef] [Green Version]
- Tasar, O.; Karabay, A.K.; Oduncu, V.; Kirma, C. Predictors and outcomes of no-reflow phenomenon in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Coron. Artery Dis. 2019, 30, 270–276. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, H.W.; Wang, C.F.; Zhang, X.J.; Tao, J.; Cui, C.S.; Meng, Q.K.; Zhu, Y.; Luo, D.F.; Hou, A.J.; et al. Incidence, predictors, and prognosis of coronary slow-flow and no-reflow phenomenon in patients with chronic total occlusion who underwent percutaneous coronary intervention. Ther. Clin. Risk Manag. 2020, 16, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Reffelmann, T.; Kloner, R.A. The “no-reflow” phenomenon: Basic science and clinical correlates. Heart 2002, 87, 162–168. [Google Scholar] [CrossRef] [Green Version]
- Bouleti, C.; Mewton, N.; Germain, S. The no-reflow phenomenon: State of the art. Arch. Cardiovasc. Dis. 2015, 108, 661–674. [Google Scholar] [CrossRef] [Green Version]
- Schofer, J.; Montz, R.; Mathey, D.G. Scintigraphic evidence of the “No reflow” phenomenon in human beings after coronary thrombolysis. J. Am. Coll. Cardiol. 1985, 5, 593–598. [Google Scholar] [CrossRef] [Green Version]
- Porter, T.R.; Li, S.; Oster, R.; Deligonul, U. The clinical implications of no reflow demonstrated with intravenous perfluorocarbon containing microbubbles following restoration of Thrombolysis In Myocardial Infarction (TIMI) 3 flow in patients with acute myocardial infarction. Am. J. Cardiol. 1998, 82, 1173–1177. [Google Scholar] [CrossRef]
- Birnbaum, Y.; Nikus, K.; Kligfield, P.; Fiol, M.; Barrabés, J.A.; Sionis, A.; Pahlm, O.; Garcia Niebla, J.; Bayès De Luna, A. The role of the ECG in diagnosis, risk estimation, and catheterization laboratory activation in patients with acute coronary syndromes: A consensus document. Ann. Noninvasive Electrocardiol. 2014, 19, 412–425. [Google Scholar] [CrossRef]
- Ding, S.; Zhao, H.; Qiao, Z.Q.; Yang, F.; Wang, W.; Gao, L.C.; Kong, L.C.; Xu, R.-D.; Ge, H.; Shen, X.D.; et al. Early resolution of ST-segment elevation after reperfusion therapy for acute myocardial infarction: Its relation to echocardiography-determined left ventricular global and regional function and deformation. J. Electrocardiol. 2015, 48, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Van’t Hof, A.W.J.; Liem, A.; De Boer, M.J.; Zijlstra, F. Clinical value of 12-lead electrocardiogram after successful reperfusion therapy for acute myocardial infarction. Lancet 1997, 350, 615–619. [Google Scholar] [CrossRef]
- Haager, P.K.; Christott, P.; Heussen, N.; Lepper, W.; Hanrath, P.; Hoffmann, R. Prediction of clinical outcome after mechanical revascularization in acute myocardial infarction by markers of myocardial reperfusion. J. Am. Coll. Cardiol. 2003, 41, 532–538. [Google Scholar] [CrossRef] [Green Version]
- Nijveldt, R.; Beek, A.M.; Hirsch, A.; Stoel, M.G.; Hofman, M.B.M.; Umans, V.A.W.M.; Algra, P.R.; Twisk, J.W.R.; van Rossum, A.C. Functional recovery after acute myocardial infarction: Comparison between angiography, electrocardiography, and cardiovascular magnetic resonance measures of microvascular injury. J. Am. Coll. Cardiol. 2008, 52, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Santoro, G.M.; Valenti, R.; Buonamici, P.; Bolognese, L.; Cerisano, G.; Moschi, G.; Trapani, M.; Antoniucci, D.; Fazzini, P.F. Relation between ST-segment changes and myocardial perfusion evaluated by myocardial contrast echocardiography in patients with acute myocardial infarction treated with direct angioplasty. Am. J. Cardiol. 1998, 82, 932–937. [Google Scholar] [CrossRef]
- Wehrens, X.H.T.; Doevendans, P.A.; Oude Ophuis, T.J.; Wellens, H.J.J. A comparison of electrocardiographic changes during reperfusion of acute myocardial infarction by thrombolysis or percutaneous transluminal coronary angioplasty. Am. Heart J. 2000, 139, 430–436. [Google Scholar] [CrossRef]
- Chesebro, J.H.; Knatterud, G.; Roberts, R.; Borer, J.; Cohen, L.S.; Dalen, J.; Dodge, H.T.; Francis, C.K.; Hillis, D.; Ludbrook, P. Thrombolysis in Myocardial Infarction (TIMI) Trial, Phase I: A comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation 1987, 76, 142–154. [Google Scholar] [CrossRef] [Green Version]
- Ndrepepa, G.; Tiroch, K.; Keta, D.; Fusaro, M.; Seyfarth, M.; Pache, J.; Mehilli, J.; Schömig, A.; Kastrati, A. Predictive factors and impact of no reflow after primary percutaneous coronary intervention in patients with acute myocardial infarction. Circ. Cardiovasc. Interv. 2010, 3, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Grygier, M.; Araszkiewicz, A.; Lesiak, M.; Janus, M.; Kowal, J.; Skorupski, W.; Pyda, M.; Mitkowski, P.; Grajek, S. New method of intracoronary adenosine injection to prevent microvascular reperfusion injury in patients with acute myocardial infarction undergoing percutaneous coronary intervention. Am. J. Cardiol. 2011, 107, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Niccoli, G.; Giubilato, S.; Russo, E.; Spaziani, C.; Leo, A.; Porto, I.; Leone, A.M.; Burzotta, F.; Riondino, S.; Pulcinelli, F.; et al. Plasma levels of thromboxane A2 on admission are associated with no-reflow after primary percutaneous coronary intervention. Eur. Heart J. 2008, 29, 1843–1850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niccoli, G.; Lanza, G.A.; Shaw, S.; Romagnoli, E.; Gioia, D.; Burzotta, F.; Trani, C.; Mazzari, M.A.; Mongiardo, R.; De Vita, M.; et al. Endothelin-1 and acute myocardial infarction: A no-reflow mediator after successful percutaneous myocardial revascularization. Eur. Heart J. 2006, 27, 1793–1798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogt, A.; von Essen, R.; Tebbe, U.; Feuerer, W.; Appel, K.F.; Niederer, W.; Neuhaus, K.L. Frequency of achieving optimal reperfusion with thrombolysis in acute myocardial infarction (analysis of four German multicenter studies). Am. J. Cardiol. 1994, 74, 1–4. [Google Scholar] [CrossRef]
- Zijlstra, F.; de Boer, M.J.; Hoorntje, J.; Reiffers, S.; Reiber, J.; Suryapranata, H. A comparison of immediate coronary angioplasty with intravenous streptokinase in acute myocardial infarction. N. Engl. J. Med. 1993, 328, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Simes, R.J.; Topol, E.J.; Holmes, D.R.; White, H.D.; Rutsch, W.R.; Vahanian, A.; Simoons, M.L.; Morris, D.; Betriu, A.; Califf, R.M.; et al. Link between the angiographic substudy and mortality outcomes in a large randomized trial of myocardial reperfusion. Importance of early and complete infarct artery reperfusion. GUSTO-I investigators. Circulation 1995, 91, 1923–1928. [Google Scholar] [CrossRef]
- Yano, A.; Ito, H.; Iwakura, K.; Kimura, R.; Tanaka, K.; Okamura, A.; Kawano, S.; Masuyama, T.; Fujii, K. Myocardial contrast echocardiography with a new calibration method can estimate myocardial viabilityin patients with myocardial infarction. J. Am. Coll. Cardiol. 2004, 43, 1799–1806. [Google Scholar] [CrossRef] [Green Version]
- Karagounis, L.; Sorensen, S.G.; Menlove, R.L.; Moreno, F.; Anderson, J.L. Does thrombolysis in myocardial infarction (TIMI) perfusion grade 2 represent a mostly patent artery or a mostly occluded artery? Enzymatic and electrocardiographic evidence from the TEAM-2 study. Second Multicenter Thrombolysis Trial of Eminase in Acute Myocardial Infarction. J. Am. Coll. Cardiol. 1992, 19, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Lyu, L.; Yang, W.; Pan, J.; Dong, M.; Zhang, M.; Zhang, P. Identification of flow-limiting coronary stenosis with PCS: A new cost-effective index derived from the product of corrected TIMI frame count and percent diameter stenosis. Front. Cardiovasc. Med. 2021, 8, 718935. [Google Scholar] [CrossRef]
- Gibson, C.M.; Murphy, S.A.; Rizzo, M.J.; Ryan, K.A.; Marble, S.J.; McCabe, C.H.; Cannon, C.P.; Van De Werf, F.; Braunwald, E. Relationship between TIMI frame count and clinical outcomes after thrombolytic administration. Thrombolysis in Myocardial Infarction (TIMI) study group. Circulation 1999, 99, 1945–1950. [Google Scholar] [CrossRef]
- Yen, C.H.; Yen, H.I.; Hou, C.J.Y.; Chou, Y.S.; Tsai, C.H. Thrombolysis in Myocardial Infarction frame count in single-vessel disease after angioplasty. Int. J. Gerontol. 2007, 1, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Van’t Hof, A.W.J.; Liem, A.; Suryapranata, H.; Hoorntje, J.C.A.; De Boer, M.J.; Zijlstra, F. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: Myocardial blush grade. Zwolle Myocardial infarction study group. Circulation 1998, 97, 2302–2306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kampinga, M.A.; Nijsten, M.W.N.; Gu, Y.L.; Dijk, W.A.; De Smet, B.J.G.L.; Van Den Heuvel, A.F.M.; Tan, E.S.; Zijlstra, F. Is the myocardial blush grade scored by the operator during primary percutaneous coronary intervention of prognostic value in patients with ST-elevation myocardial infarction in routine clinical practice? Circ. Cardiovasc. Interv. 2010, 3, 216–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorajja, P.; Gersh, B.J.; Costantini, C.; McLaughlin, M.G.; Zimetbaum, P.; Cox, D.A.; Garcia, E.; Tcheng, J.E.; Mehran, R.; Lansky, A.J.; et al. Combined prognostic utility of ST-segment recovery and myocardial blush after primary percutaneous coronary intervention in acute myocardial infarction. Eur. Heart J. 2005, 26, 667–674. [Google Scholar] [CrossRef]
- Gibson, C.M.; Cannon, C.P.; Murphy, S.A.; Ryan, K.A.; Mesley, R.; Marble, S.J.; McCabe, C.H.; Van De Werf, F.; Braunwald, E. Relationship of TIMI myocardial perfusion grade to mortality after administration of thrombolytic drugs. Circulation 2000, 101, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Gibson, C.M.; Cannon, C.P.; Murphy, S.A.; Marble, S.J.; Barron, H.V.; Braunwald, E. Relationship of the TIMI myocardial perfusion grades, flow grades, frame count, and percutaneous coronary intervention to long-term outcomes after thrombolytic administration in acute myocardial infarction. Circulation 2002, 105, 1909–1913. [Google Scholar] [CrossRef] [Green Version]
- Carrick, D.; Oldroyd, K.G.; McEntegart, M.; Haig, C.; Petrie, M.C.; Eteiba, H.; Hood, S.; Owens, C.; Watkins, S.; Layland, J.; et al. A randomized trial of deferred stenting versus immediate stenting to prevent no- or slow-reflow in acute ST-segment elevation myocardial infarction (DEFER-STEMI). J. Am. Coll. Cardiol. 2014, 63, 2088–2098. [Google Scholar] [CrossRef] [Green Version]
- Wong, D.T.L.; Leung, M.C.H.; Richardson, J.D.; Puri, R.; Bertaso, A.G.; Williams, K.; Meredith, I.T.; Teo, K.S.L.; Worthley, M.I.; Worthley, S.G. Cardiac magnetic resonance derived late microvascular obstruction assessment post ST-segment elevation myocardial infarction is the best predictor of left ventricular function: A comparison of angiographic and cardiac magnetic resonance derived measurements. Int. J. Cardiovasc. Imaging 2012, 28, 1971–1981. [Google Scholar] [CrossRef]
- De Waha, S.; Patel, M.R.; Granger, C.B.; Ohman, E.M.; Maehara, A.; Eitel, I.; Ben-Yehuda, O.; Jenkins, P.; Thiele, H.; Stone, G.W. Relationship between microvascular obstruction and adverse events following primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: An individual patient data pooled analysis from seven randomized trials. Eur. Heart J. 2017, 38, 3502–3510. [Google Scholar] [CrossRef]
- Bolognese, L.; Carrabba, N.; Parodi, G.; Santoro, G.M.; Buonamici, P.; Cerisano, G.; Antoniucci, D. Impact of microvascular dysfunction on left ventricular remodeling and long-term clinical outcome after primary coronary angioplasty for acute myocardial infarction. Circulation 2004, 109, 1121–1126. [Google Scholar] [CrossRef]
- Wu, K.C.; Kim, R.J.; Bluemke, D.A.; Rochitte, C.E.; Zerhouni, E.A.; Becker, L.C.; Lima, J.A.C. Quantification and time course of microvascular obstruction by contrast-enhanced echocardiography and magnetic resonance imaging following acute myocardial infarction and reperfusion. J. Am. Coll. Cardiol. 1998, 32, 1756–1764. [Google Scholar] [CrossRef] [Green Version]
- Lund, G.K.; Stork, A.; Saeed, M.; Bansmann, M.P.; Gerken, J.H.; Müller, V.; Mester, J.; Higgins, C.B.; Adam, G.; Meinertz, T. Acute myocardial infarction: Evaluation with first-pass enhancement and delayed enhancement MR imaging compared with 201Tl SPECT imaging. Radiology 2004, 232, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Bekkers, S.C.A.M.; Backes, W.H.; Kim, R.J.; Snoep, G.; Gorgels, A.P.M.; Passos, V.L.; Waltenberger, J.; Crijns, H.J.G.M.; Schalla, S. Detection and characteristics of microvascular obstruction in reperfused acute myocardial infarction using an optimized protocol for contrast-enhanced cardiovascular magnetic resonance imaging. Eur. Radiol. 2009, 19, 2904–2912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Waha, S.; Desch, S.; Eitel, I.; Fuernau, G.; Zachrau, J.; Leuschner, A.; Gutberlet, M.; Schuler, G.; Thiele, H. Impact of early vs. late microvascular obstruction assessed by magnetic resonance imaging on long-term outcome after ST-elevation myocardial infarction: A comparison with traditional prognostic markers. Eur. Heart J. 2010, 31, 2660–2668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, D.T.L.; Leung, M.C.H.; Das, R.; Liew, G.Y.H.; Teo, K.S.L.; Chew, D.P.; Meredith, I.T.; Worthley, M.I.; Worthley, S.G. Intracoronary ECG during primary percutaneous coronary intervention for ST-segment elevation myocardial infarction predicts microvascular obstruction and infarct size. Int. J. Cardiol. 2013, 165, 61–66. [Google Scholar] [CrossRef]
- Mather, A.N.; Fairbairn, T.A.; Artis, N.J.; Greenwood, J.P.; Plein, S. Timing of cardiovascular MR imaging after acute myocardial infarction: Effect on estimates of infarct characteristics and prediction of late ventricular remodeling. Radiology 2011, 261, 116–126. [Google Scholar] [CrossRef] [Green Version]
- Jayaweera, A.R.; Edwards, N.; Glasheen, W.P.; Villanueva, F.S.; Abbott, R.D.; Kaul, S. In vivo myocardial kinetics of air-filled albumin microbubbles during myocardial contrast echocardiography. Comparison with radiolabeled red blood cells. Circ. Res. 1994, 74, 1157–1165. [Google Scholar] [CrossRef] [Green Version]
- Kaul, S.; Villanueva, F.S. Is the determination of myocardial perfusion necessary to evaluate the success of reperfusion when the infarct-related artery is open? Circulation 1992, 85, 1942–1944. [Google Scholar] [CrossRef] [Green Version]
- Swinburn, J.M.A.; Lahiri, A.; Senior, R. Intravenous myocardial contrast echocardiography predicts recovery of dysynergic myocardium early after acute myocardial infarction. J. Am. Coll. Cardiol. 2001, 38, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Agati, L.; Tonti, G.; Pedrizzetti, G.; Magri, F.; Funaro, S.; Madonna, M.; Celani, F.; Messager, T.; Broillet, A. Clinical application of quantitative analysis in real-time MCE. Eur. J. Echocardiogr. 2004, 5, S17–S23. [Google Scholar] [CrossRef] [Green Version]
- Ito, H.; Tomooka, T.; Sakai, N.; Yu, H.; Higashino, Y.; Fujii, K.; Masuyama, T.; Kitabatake, A.; Minamino, T. Lack of myocardial perfusion immediately after successful thrombolysis. A predictor of poor recovery of left ventricular function in anterior myocardial infarction. Circulation 1992, 85, 1699–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayat, S.A.; Senior, R. Myocardial contrast echocardiography in ST elevation myocardial infarction: Ready for prime time? Eur. Heart J. 2008, 29, 299–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomaszuk-Kazberuk, A.; Sobkowicz, B.; Kaminski, K.; Gugala, K.; Mezynski, G.; Dobrzycki, S.; Lewczuk, A.; Kazberuk, W.; Musial, W.J. Myocardial perfusion assessed by contrast echocardiography correlates with angiographic perfusion parameters in patients with a first acute myocardial infarction successfully treated with angioplasty. Can. J. Cardiol. 2008, 24, 633–639. [Google Scholar] [CrossRef] [Green Version]
- Tong, K.L.; Kaul, S.; Wang, X.Q.; Rinkevich, D.; Kalvaitis, S.; Belcik, T.; Lepper, W.; Foster, W.A.; Wei, K. Myocardial contrast echocardiography versus Thrombolysis in Myocardial Infarction score in patients presenting to the emergency department with chest pain and a nondiagnostic electrocardiogram. J. Am. Coll. Cardiol. 2005, 46, 920–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinkevich, D.; Kaul, S.; Wang, X.Q.; Khim, L.T.; Belcik, T.; Kalvaitis, S.; Lepper, W.; Dent, J.M.; Wei, K. Regional left ventricular perfusion and function in patients presenting to the emergency department with chest pain and no ST-segment elevation. Eur. Heart J. 2005, 26, 1606–1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, Y.; Kumita, S.I.; Cho, K.; Toba, M.; Mizumura, S.; Tanaka, K.; Takano, T.; Kumazaki, T. Evaluation of no-reflow phenomenon using 201TlCl/123I-BMIPP dual-isotope myocardial SPECT. J. Nippon Med. Sch. 2006, 73, 258–264. [Google Scholar] [CrossRef] [Green Version]
- Hamada, S.; Nakamura, S.; Sugiura, T.; Murakami, T.; Fujimoto, T.; Watanabe, J.; Baden, M.; Hatada, K.; Iwasaka, T. Early detection of the no-reflow phenomenon in reperfused acute myocardial infarction using technetium-99m tetrofosmin imaging. Eur. J. Nucl. Med. 1999, 26, 208–214. [Google Scholar] [CrossRef]
- Kloner, R.A.; Ganote, C.E.; Jennings, R.B. The “no-reflow” phenomenon after temporary coronary occlusion in the dog. J. Clin. Investig. 1974, 54, 1496–1508. [Google Scholar] [CrossRef] [Green Version]
- Pelletier-Galarneau, M.; Martineau, P.; El Fakhri, G. Quantification of PET myocardial blood flow. Curr. Cardiol. Rep. 2019, 21, 11. [Google Scholar] [CrossRef]
- Jaarsma, C.; Leiner, T.; Bekkers, S.C.; Crijns, H.J.; Wildberger, J.E.; Nagel, E.; Nelemans, P.J.; Schalla, S. Diagnostic performance of noninvasive myocardial perfusion imaging using single-photon emission computed tomography, cardiac magnetic resonance, and positron emission tomography imaging for the detection of obstructive coronary artery disease: A meta-analysis. J. Am. Coll. Cardiol. 2012, 59, 1719–1728. [Google Scholar] [CrossRef] [Green Version]
- Akutsu, Y.; Kaneko, K.; Kodama, Y.; Li, H.L.; Nishimura, H.; Hamazaki, Y.; Suyama, J.; Shinozuka, A.; Gokan, T.; Kobayashi, Y. Technetium-99m pyrophosphate/thallium-201 dual-isotope SPECT imaging predicts reperfusion injury in patients with acute myocardial infarction after reperfusion. Eur. J. Nucl. Med. Mol. Imaging 2008, 36, 230–236. [Google Scholar] [CrossRef]
- Henzlova, M.J.; Duvall, W.L.; Einstein, A.J.; Travin, M.I.; Verberne, H.J. ASNC imaging guidelines for SPECT nuclear cardiology procedures: Stress, protocols, and tracers. J. Nucl. Cardiol. 2016, 23, 606–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iskandrian, A.S. Single-photon emission computed tomographic thallium imaging with adenosine, dipyridamole, and exercise. Am. Heart J. 1991, 122, 279–284. [Google Scholar] [CrossRef]
- Arbab-Zadeh, A.; Carli, M.F.D.; Cerci, R.; George, R.T.; Chen, M.Y.; Dewey, M.; Niinuma, H.; Vavere, A.L.; Betoko, A.; Plotkin, M.; et al. Accuracy of CT angiography and SPECT myocardial perfusion imaging for the diagnosis of coronary artery disease. Circ. Cardiovasc. Imaging 2015, 8, e003533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Wu, J.; Miller, E.J.; Liu, C.; Liu, Y.; Liu, Y.H. Diagnostic accuracy of stress-only myocardial perfusion SPECT improved by deep learning. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2793–2800. [Google Scholar] [CrossRef]
- Lameka, K.; Farwell, M.D.; Ichise, M. Positron emission tomography. Handb. Clin. Neurol. 2016, 135, 209–227. [Google Scholar] [CrossRef] [Green Version]
- Maes, A.; Van De Werf, F.; Nuyts, J.; Bormans, G.; Desmet, W.; Mortelmans, L. Impaired myocardial tissue perfusion early after successful thrombolysis. Circulation 1995, 92, 2072–2078. [Google Scholar] [CrossRef]
- Desmet, W.J.; Mesotten, L.V.; Maes, A.F.; Heidbüchel, H.P.; Mortelmans, L.A.; Van De Werf, F.J. Relation between different methods for analysing ST segment deviation and infarct size as assessed by positron emission tomography. Heart 2004, 90, 887–892. [Google Scholar] [CrossRef] [Green Version]
- Salustri, A.; Pozzoli, M.M.A.; Ilmer, B.; Hermans, W.; Reijs, A.E.M.; Reiber, J.H.C.; Roelandt, J.R.T.C.; Fioretti, P.M. Exercise echocardiography and single photon emission computed tomography in patients with left anterior descending coronary artery stenosis. Int. J. Card. Imaging 1992, 8, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Driessen, R.S.; Raijmakers, P.G.; Stuijfzand, W.J.; Knaapen, P. Myocardial perfusion imaging with PET. Int. J. Cardiovasc. Imaging 2017, 33, 1021–1031. [Google Scholar] [CrossRef] [Green Version]
- Claeys, M.J.; Bosmans, J.; Veenstra, L.; Jorens, P.; De Raedt, H.; Vrints, C.J. Determinants and prognostic implications of persistent ST-segment elevation after primary angioplasty for acute myocardial infarction importance of microvascular reperfusion injury on clinical outcome. Circulation 1999, 99, 1972–1977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamaki, N.; Kawamoto, M.; Tadamura, E.; Magata, Y.; Yonekura, Y.; Nohara, R.; Sasayama, S.; Nishimura, K.; Ban, T.; Konishi, J. Prediction of reversible ischemia after revascularization. Perfusion and metabolic studies with positron emission tomography. Circulation 1995, 91, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Caiazzo, G.; Musci, R.L.; Frediani, L.; Umińska, J.; Wanha, W.; Filipiak, K.J.; Kubica, J.; Navarese, E.P. State of the art: No-reflow phenomenon. Cardiol. Clin. 2020, 38, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Tatu-Chițoiu, G. Electrocardiograma in Reperfuzia Miocardica; Editura Medicală Antaeus: Bucharest, Romania, 2014; ISBN 606-8470-08-5. [Google Scholar]
- Giugliano, R.P.; Sabatine, M.S.; Gibson, C.M.; Roe, M.T.; Harrington, R.A.; Murphy, S.A.; Morrow, D.A.; Antman, E.M.; Braunwald, E. Combined assessment of thrombolysis in myocardial infarction flow grade, myocardial perfusion grade, and ST-segment resolution to evaluate epicardial and myocardial reperfusion. Am. J. Cardiol. 2004, 93, 1362–1367. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Corbett, S.; Chettibi, M.; Hayrapetyan, H.; et al. Fourth universal definition of myocardial infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef]
- Khan, R.; Zarak, M.S.; Munir, U.; Ahmed, K.; Ullah, A. Thrombolysis in Myocardial Infarction (TIMI) risk score assessment for complications in acute anterior wall ST elevation myocardial infarction. Cureus 2020, 12, e8646. [Google Scholar] [CrossRef]
- Seyfeli, E.; Abaci, A.; Kula, M.; Topsakal, R.; Eryol, N.K.; Arinc, H.; Ozdogru, I.; Ergin, A. Myocardial blush grade: To evaluate myocardial viability in patients with acute myocardial infarction. Angiology 2007, 58, 556–560. [Google Scholar] [CrossRef]
- Bauer, T.; Zeymer, U.; Diallo, A.; Vicaut, E.; Bolognese, L.; Cequier, A.; Huber, K.; Montalescot, G.; Hamm, C.W.; van’t Hof, A.W. Impact of preprocedural TIMI flow on clinical outcome in low-risk patients with ST-elevation myocardial infarction: Results from the ATLANTIC study. Catheter. Cardiovasc. Interv. 2020, 95, 494–500. [Google Scholar] [CrossRef]
- Kaya, M.G.; Arslan, F.; Abaci, A.; Van Der Heijden, G.; Timurkay-Nak, T.; Cengel, A. Myocardial blush grade: A predictor for major adverse cardiac events after primary PTCA with stent implantation for acute myocardial infarction. Acta Cardiol. 2007, 62, 445–451. [Google Scholar] [CrossRef]
- Kaul, S. Evaluating the “no reflow” phenomenon with myocardial contrast echocardiography. Basic Res. Cardiol. 2006, 101, 391–399. [Google Scholar] [CrossRef]
- Wu, K.C. CMR of microvascular obstruction and hemorrhage in myocardial infarction. J. Cardiovasc. Magn. Reson. 2012, 14, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeremy, R.W.; Links, J.M.; Becker, L.C. Progressive failure of coronary flow during reperfusion of myocardial infarction: Documentation of the no reflow phenomenon with positron emission tomography. J. Am. Coll. Cardiol. 1990, 16, 695–704. [Google Scholar] [CrossRef] [Green Version]





| Patients (No.) | Diagnostic Method | Incidence % | Ref. | ||
|---|---|---|---|---|---|
| STEMI | No-Reflow | Normal Flow | |||
| 126 | 47 | 79 | MCE | 37 | Ito et al., 1996 [16] |
| 1658 | 491 | 1167 | PCI | 42 | Yang et al., 2020 [17] |
| 5997 | 128 | 5869 | 2.1 | Cenko et al., 2016 [18] | |
| 203 | 38 | 165 | 18.7 | Li et al., 2018 [19] | |
| 291,380 | 6553 | 284,827 | 2.3 | Harrison et al., 2013 [5] | |
| 93 | 28 | 65 | 30 | Morishima et al.,1995 [20] | |
| 126 | 47 | 79 | 37 | Ito et al., 1996 [16] | |
| 143 | 24 | 119 | 13.9 | Rossington et al., 2020 [21] | |
| 733 | 54 | 679 | 16.1 | Liang et al., 2017 [22] | |
| 347 | 110 | 237 | 32 | Rezkalla et al., 2010 [23] | |
| 100 | 27 | 73 | 2.7 | Mahmoud et al., 2019 [24] | |
| 44 | 11 | 33 | MRI | 25 | Wu et al., 1998 [25] |
| Diagnostic Methods | Characteristic, Observations | Ref. |
|---|---|---|
| Classical | ||
| ECG |
| Caiazzo et al., 2020, Tatu-Chițoiu et al., 2014 [113,114] |
| Coronary angiography |
| Gupta et al., 2016 Sorraja et al., 2005 Thygesen et al., 2018 Khan et al., 2020 Seyfeli et al., 2007 Bauer et al., 2020 Kaya et al., 2007 Giugliano et al., 2004 [27,74,115,116,117,118,119,120] |
| Modern | ||
| MCE |
| Kaul et al., 2006 [121] |
| Intracardiac Echocardiography |
| Ramjane et al., 2008 [4] |
| CMRI |
| Wu et al., 2012 [122] |
| SPECT |
| Shimizu et al., 2006 [96] |
| PET |
| Jeremy et al., 1990 [123] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pantea-Roșan, L.R.; Bungau, S.G.; Radu, A.-F.; Pantea, V.A.; Moisi, M.I.; Vesa, C.M.; Behl, T.; Nechifor, A.C.; Babes, E.E.; Stoicescu, M.; et al. A Narrative Review of the Classical and Modern Diagnostic Methods of the No-Reflow Phenomenon. Diagnostics 2022, 12, 932. https://doi.org/10.3390/diagnostics12040932
Pantea-Roșan LR, Bungau SG, Radu A-F, Pantea VA, Moisi MI, Vesa CM, Behl T, Nechifor AC, Babes EE, Stoicescu M, et al. A Narrative Review of the Classical and Modern Diagnostic Methods of the No-Reflow Phenomenon. Diagnostics. 2022; 12(4):932. https://doi.org/10.3390/diagnostics12040932
Chicago/Turabian StylePantea-Roșan, Larisa Renata, Simona Gabriela Bungau, Andrei-Flavius Radu, Vlad Alin Pantea, Mădălina Ioana Moisi, Cosmin Mihai Vesa, Tapan Behl, Aurelia Cristina Nechifor, Elena Emilia Babes, Manuela Stoicescu, and et al. 2022. "A Narrative Review of the Classical and Modern Diagnostic Methods of the No-Reflow Phenomenon" Diagnostics 12, no. 4: 932. https://doi.org/10.3390/diagnostics12040932
APA StylePantea-Roșan, L. R., Bungau, S. G., Radu, A. -F., Pantea, V. A., Moisi, M. I., Vesa, C. M., Behl, T., Nechifor, A. C., Babes, E. E., Stoicescu, M., Gitea, D., Iovanovici, D. C., & Bustea, C. (2022). A Narrative Review of the Classical and Modern Diagnostic Methods of the No-Reflow Phenomenon. Diagnostics, 12(4), 932. https://doi.org/10.3390/diagnostics12040932