Reduction of Specular Reflection Based on Linear Polarization Control for Fluorescence-Induced Diagnostic Evaluation
Abstract
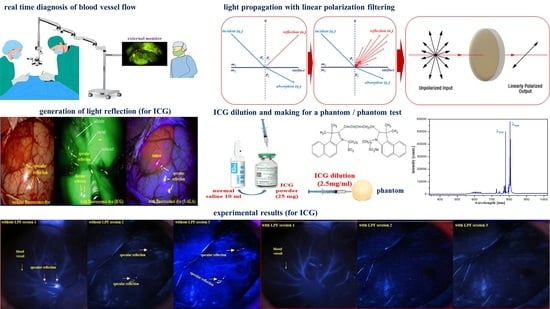
:1. Introduction
2. Analysis of Fluorescence Emission and Specular Reflection
3. How to Eliminate Occurrence of Specular Reflection
4. Experiment Configuration and Results
4.1. Experiment Configuration
4.1.1. Phantom Production
4.1.2. Phantom Reagent Production
4.1.3. Experiment Device Configuration
4.2. Experiment Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hosmann, A.; Millesi, M.; Wadiura, L.I.; Kiesel, B.; Mercea, P.A.; Mischkulnig, M.; Borkovec, M.; Furtner, J.; Roetzer, T.; Wolfsberger, S.; et al. 5-ALA Fluorescence is a Powerful Prognostic Marker During Surgery of Low-Grade Gliomas (WHO Grade II)—Experience at Two Specialized Centers. Cancers 2021, 13, 2540. [Google Scholar] [CrossRef] [PubMed]
- Sabel, M.; Knipps, J.; Neumann, L.M.; Kieslich, M.; Steiger, H.-J.; Rapp, M.; Kamp, M.A. Quantification of ALA-fluorescence induced by a modified commercially available head lamp and a surgical microscope. Neurosurg. Rev. 2018, 41, 1079–1083. [Google Scholar] [CrossRef] [PubMed]
- Desmettre, T.; Devoisselle, J.; Mordon, S. Fluorescence Properties and Metabolic Features of Indocyanine Green (ICG) as Related to Angiography. Surv. Ophthalmol. 2000, 45, 15–27. [Google Scholar] [CrossRef]
- Hong, N.Y.; Kim, H.R.; Lee, H.M.; Sohn, D.K.; Kim, K.G. Fluorescent property of indocyanine green (ICG) rubber ring using LED and laser light sources. Biomed. Opt. Express 2016, 7, 1637–1644. [Google Scholar] [CrossRef]
- Hadjipanayis, C.G.; Stummer, W. 5-ALA and FDA approval for glioma surgery. J. Neuro-Oncol. 2019, 141, 479–486. [Google Scholar] [CrossRef]
- Hirche, C.; Engel, H.; Kolios, L.; Cognie, J.; Hünerbein, M.; Lehnhardt, M.; Kremer, T. An Experimental Study to Evaluate the Fluobeam 800 Imaging System for Fluorescence-Guided Lymphatic Imaging and Sentinel Node Biopsy. Surg. Innov. 2012, 20, 516–523. [Google Scholar] [CrossRef]
- Kim, H.R.; Lee, H.M.; Yoo, H.; Lee, S.H.; Kim, K.G. Review of Neurosurgical Fluorescence Imaging Systems for Clinical Application. J. Opt. Soc. Korea 2016, 20, 305–313. [Google Scholar] [CrossRef]
- Stummer., W.; Novotny, A.; Stepp, H.; Goetz, C.; Bise, K.; Reulen, H.J. Fluorescence-guided resection of glioblastoma muliforme utilizing 5-ALA-induced porphyrins: A prospective study in consecutive patients. J. Neurosurg. 2000, 93, 1003–1013. [Google Scholar] [CrossRef]
- Kwon, S.Y.; Yoon, K.-C.; Kim, K.G. Reduction of Reflected Light on Fluorescence Emission for Indocyanine Green. J. Eng. Sci. Med Diagn. Ther. 2022, 5, 011004. [Google Scholar] [CrossRef]
- Jiao, J.; Fan, W.; Sun, J.; Satoshi, N. Highlight removal for camera captured documents based on image stitching. In Proceedings of the 13th International Conference on Signal Processing, Santorini, Greece, 2–4 July 1997. [Google Scholar]
- Wan, R.; Shi, B.; Duan, L.Y.; Tan, A.H.; Kot, A.C. Benchmarking single image reflection removal algorithm. In Proceedings of the International Conference on Computer Vision, Venice, Italy, 22–29 October 2017. [Google Scholar]
- Li, C.; Yang, Y.; He, K.; Lin, S.; Hopcroft, J.E. Single Image Reflection Removal Through Cascaded Refinement. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), Seattle, WA, USA, 13–19 June 2020; pp. 3565–3571. [Google Scholar] [CrossRef]
- Tchoulack, S.; Pierre Langlois, J.M.; Cheriet, F. A video stream processor for real-time detection and correction of specular reflections in endoscopic images. In Proceedings of the Joint 6th International IEEE Northeast Workshop on Circuits and Systems and TAISA Conference, Montreal, QC, Canada, 22–25 June 2008; pp. 49–52. [Google Scholar] [CrossRef]
- Wang, J.; Eng, H.; Kam, A.H.; Yau, W. Specular reflection removal for human detection under aquatic environment. In Proceedigns of the Computer Society Conference on Computer Vision and Pattern Recognition Workshops, Washington, DC, USA, 27 June–2 July 2004. [Google Scholar]
- Yamazaki, M.; Chen, Y.; Xu, G. Separating reflections from images using kernel independent component analysis. In Proceedings of the 18th International Conference on Pattern Recognition, Hong Kong, China, 20–24 August 2006; Volume 3, pp. 194–197. [Google Scholar] [CrossRef]
- Hodgson, A.; Kelly, N.; Peel, D. Unmanned Aerial Vehicles (UAVs) for Surveying Marine Fauna: A Dugong Case Study. PLoS ONE 2013, 8, e79556. [Google Scholar] [CrossRef]
- Ahn, J.; Nishida, K.; Ishii, Y.; Ura, T. A sea creatures classification method using convolutional neural networks. In Proceedings of the IEEE 18th International Conference on Control, Automation and Systems (ICCAS), PyeongChang, Korea, 17–20 October 2018. [Google Scholar]
- So, H.-R.; Oh, S.-J.; Jin, G.-G. Modified Geometric Step-Based Box-Counting Method to Estimate the Complexity of Korean Peninsula Coastlines. J. Inst. Control. Robot. Syst. 2019, 25, 30–36. [Google Scholar] [CrossRef]
- Gonçalves, J.A.; Henriques, R. UAV photogrammetry for topographic monitoring of coastal areas. ISPRS J. Photogramm. Remote Sens. 2015, 104, 101–111. [Google Scholar] [CrossRef]
- Han, B.-J.; Sim, J.-Y. Reflection removal using low-rank matrix completion. In Proceedings of the IEEE CVPR, Honolulu, HI, USA, 21–26 July 2017; pp. 3872–3880. [Google Scholar] [CrossRef]
- Han, B.-J.; Sim, J.-Y. Glass Reflection Removal Using Co-Saliency-Based Image Alignment and Low-Rank Matrix Completion in Gradient Domain. IEEE Trans. Image Process. 2018, 27, 4873–4888. [Google Scholar] [CrossRef] [PubMed]
- Han, B.-J.; Sim, J.-Y. Single Image Reflection Removal Using Non-Linearly Synthesized Glass Images and Semantic Context. IEEE Access 2019, 7, 170796–170806. [Google Scholar] [CrossRef]
- Hardian, R.F.; Hanaoka, Y.; Funato, K.; Agata, M.; Ito, K.; Horiuchi, T. Assessment of collateral venous flow using temporary clip placement at ICG videoangiography. Acta Neurochir. 2021, 163, 2533–2536. [Google Scholar] [CrossRef]
- Goryaynov, S.; Widhalm, G.; Goldberg, M.F.; Chelushkin, D.; Spallone, A.; Chernyshov, K.A.; Ryzhova, M.; Pavlova, G.; Revischin, A.; Shishkina, L.; et al. The Role of 5-ALA in Low-Grade Gliomas and the Influence of Antiepileptic Drugs on Intraoperative Fluorescence. Front. Oncol. 2019, 22, 423. [Google Scholar] [CrossRef]
- Valli, D.; Belykh, E.; Zhao, X.; Gandhi, S.; Cavallo, C.; Martirosyan, N.L.; Nakaji, P.; Lawton, M.T.; Preul, M.C. Development of a Simulation Model for Fluorescence-Guided Brain Tumor Surgery. Front. Oncol. 2019, 16, 748. [Google Scholar] [CrossRef]
- Ramezani, S.; Parkhideh, A.; Bhattacharya, P.K.; Farach-Carson, M.C.; Harrington, D.A. Beyond Colonoscopy: Exploring New Cell Surface Biomarkers for Detection of Early, Heterogenous Colorectal Lesions. Front. Oncol. 2021, 11, 1471. [Google Scholar] [CrossRef]
- Wright, R.S.; Sweet, M. OpenGL Superbible: The Complete Guide to OpenGL Programming for Windows NT and Windows 98; Waite Group Press: Corte Madera, CA, USA, 1996. [Google Scholar]
- Kim, S.; Ra, M.; Kim, W.-Y. Specular Detection on Glossy Surface Using Geometric Characteristics of Specularity in Top-View Images. Sensors 2021, 21, 2079. [Google Scholar] [CrossRef]
- Zeng, J.; Chang, B.; Du, D.; Hong, Y.; Chang, S.; Zou, Y. A Precise Visual Method for Narrow Butt Detection in Specular Reflection Workpiece Welding. Sensors 2016, 16, 1480. [Google Scholar] [CrossRef]
- Yoon, K.; Seol, J.; Kim, K.G. Removal of Specular Reflection Using Angle Adjustment of Linear Polarized Filter in Medical Imaging Diagnosis. Diagnostics 2022, 12, 863. [Google Scholar] [CrossRef]
- de Elguea-Lizárraga, J.I.O.; Riojas-Garza, A.; Chapa-Lobo, A.; Rangel-Ríos, H.A.; García-García, A.L.; Quevedo-Fernández, E.; Salgado-Cruz, L.E. Indocyanine green fluorescence angiography in colorectal surgery. First case series in Mexico. Rev. Gastroenterol. De México 2021, 87, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Coemans, M.A.J.M.; Hzn Vos, J.J.; Nuboer, J.F.W. The orientation of the E-vector of linearly polarized light does not affect the behaviour of the pigeon columba livia. J. Exp. Biol. 1994, 191, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Muheim, R. Behavioural and physiological mechanisms of polarized light sensitivity in birds. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.; Stari, C.; Cabeza, C.; Marti, A.C. The Polarization of Light and Malus’ Law Using Smartphones. Phys. Teach. 2017, 55, 264–266. [Google Scholar] [CrossRef]
- Rosi, T.; Onorato, P. Video analysis-based experiments regarding Malus’ law. Phys. Educ. 2020, 55, 045011. [Google Scholar] [CrossRef]
- Woźniak, W.A.; Kurzynowski, P.; Zdunek, M. Malus law—Interferometric interpretation. Opt. Appl. 2013, 43, 237–246. [Google Scholar]
- Mohamcd, A.M.; Hassan, A.F. Improve solar cell energy conversion efficiency by using illuminescent dyes concentrators. J. Kufa Phys. 2015, 7, 8–15. [Google Scholar]
- Yoon, K.-C.; Kim, K.G.; Lee, S.H. Design of a Surgical Pen-Type Probe for Real-Time Indocyanine Green Fluorescence Emission Diagnosis. J. Med Devices 2021, 15, 041009. [Google Scholar] [CrossRef]
- Asif, M.; Song, H.; Chen, L.; Yang, J.; Frangi, A.F. Intrinsic layer based automatic specular reflection detection in endoscopic images. Comput. Biol. Med. 2021, 128, 104106. [Google Scholar] [CrossRef]
- Yoo, H. Closed-form least-squares technique for adaptive linear image interpolation. Electron. Lett. 2007, 43, 210–212. [Google Scholar] [CrossRef]
- Kirkpatrick, S.J.; Duncan, D.D.; Wells-Gray, E.M. Detrimental effects of speckle-pixel size matching in laser speckle contrast imaging. Opt. Lett. 2008, 33, 2886–2888. [Google Scholar] [CrossRef] [PubMed]























| Rotation Angle of the Filter F2 (θ) | Specular Reflection Intensity [mW/cm2] | Rotation Angle of Filter F2 (θ) | Specular Reflection Intensity [mW/cm2] |
|---|---|---|---|
| 0° | 50.0 | 210° | 37.5 |
| 30° | 37.5 | 240° | 12.5 |
| 60° | 50.0 | 270° | 0.00 |
| 90° | 0.00 | 300° | 12.5 |
| 120° | 12.5 | 330° | 37.5 |
| 150° | 37.5 | 360° | 50.0 |
| 180° | 50.0 |
| Performance (@ LED) | Parameter | Performance (@ Camera) | Parameter |
|---|---|---|---|
| model | Thorlabs LED 780E | model | SJ-8200 |
| wavelength, λ (nm) | 780–785 | sensor | CMOS |
| output power (mW) | 18.0 | Resolution [P] | 1920 |
| current (mA) | 100 | pixel size [Mpixel] | 2.0 |
| voltage (V) | 1.75–1.95 | frame rete [fps] | 30 |
| beam angle of radiation, θ (deg) | 10 | focal distance | 5 mm–infinity |
| luminous intensity (mrcd) | 2500 | view angle [deg] | 60° |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Yoon, K.; Kim, J.; Kim, K.G. Reduction of Specular Reflection Based on Linear Polarization Control for Fluorescence-Induced Diagnostic Evaluation. Diagnostics 2022, 12, 1990. https://doi.org/10.3390/diagnostics12081990
Lee S, Yoon K, Kim J, Kim KG. Reduction of Specular Reflection Based on Linear Polarization Control for Fluorescence-Induced Diagnostic Evaluation. Diagnostics. 2022; 12(8):1990. https://doi.org/10.3390/diagnostics12081990
Chicago/Turabian StyleLee, Sangyun, Kicheol Yoon, Jungmin Kim, and Kwang Gi Kim. 2022. "Reduction of Specular Reflection Based on Linear Polarization Control for Fluorescence-Induced Diagnostic Evaluation" Diagnostics 12, no. 8: 1990. https://doi.org/10.3390/diagnostics12081990
APA StyleLee, S., Yoon, K., Kim, J., & Kim, K. G. (2022). Reduction of Specular Reflection Based on Linear Polarization Control for Fluorescence-Induced Diagnostic Evaluation. Diagnostics, 12(8), 1990. https://doi.org/10.3390/diagnostics12081990