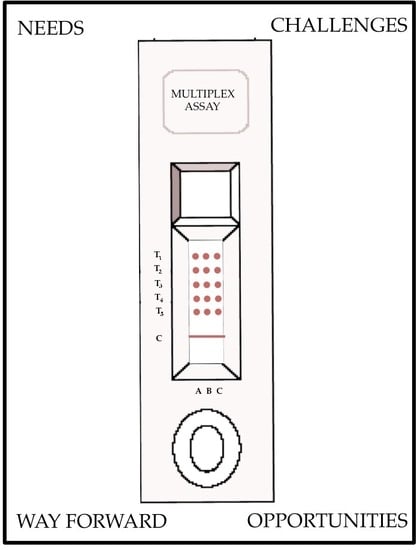
Development of Multiplexed Infectious Disease Lateral Flow Assays: Challenges and Opportunities
Abstract
:1. Needs and Impetus for Multiplexed Lateral Flow
2. Challenges and Emerging Technologies in Multiplexing LFAs
3. Way Forward
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Mohd Hanafiah, K.; Garcia, M.; Anderson, D. Point-of-care testing and the control of infectious diseases. Biomark. Med. 2013, 7, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Guo, J.; Wang, X. Immunochromatographic lateral flow strip tests. Methods Mol. Biol. 2009, 504, 169–183. [Google Scholar] [PubMed]
- Weidemaier, K.; Carrino, J.; Curry, A.; Connor, J.H.; Liebmann-Vinson, A. Advancing Rapid Point-of-care Viral Diagnostics to a Clinical Setting. Future Virol. 2015, 10, 313–328. [Google Scholar] [CrossRef]
- Marquez, P.V.; Farrington, J.L. No more disease silos for sub-Saharan Africa. BMJ 2012, 345, e5812. [Google Scholar] [CrossRef] [PubMed]
- Bottcher, L.; Woolley-Meza, O.; Araújo, N.A.M.; Herrmann, H.J.; Helbing, D. Disease-induced resource constraints can trigger explosive epidemics. Sci. Rep. 2015, 5, 16571. [Google Scholar] [CrossRef] [PubMed]
- Omilabu, S.A.; Salu, O.B.; Oke, B.O.; James, A.B. The West African ebola virus disease epidemic 2014–2015: A commissioned review. Niger. Postgrad. Med. J. 2016, 23, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, F.; Damkjaer, M.; Lunding, S.; Dornonville de la Cour, K.; Young, A.; Brooks, T.; Sesay, T.; Salam, A.P.; Mishra, S.; Storgaard, M. Influence of Referral Pathway on Ebola Virus Disease Case-Fatality Rate and Effect of Survival Selection Bias. Emerg. Infect. Dis. 2017, 23, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Baumann, R.; Kaempfer, S.; Chegou, N.N.; Oehlmann, W.; Loxton, A.G.; Kaufmann, S.H.; van Helden, P.D.; Black, G.F.; Singh, M.; Walzl, G. Serologic diagnosis of tuberculosis by combining Ig classes against selected mycobacterial targets. J. Infect. 2014, 69, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Mohd Hanafiah, K.; Liu, J.J.; Lieschke, K.; Barnes, N.C.; Garcia, M.L.; Anderson, D.A. Serological biomarker screening and host factor analysis elucidating immune response heterogeneity in active pulmonary tuberculosis. Trop. Biomed. 2017, 34, 1–14. [Google Scholar]
- Luka, G.; Ahmadi, A.; Najjaran, H.; Alocilja, E.; DeRosa, M.; Wolthers, K.; Malki, A.; Aziz, H.; Althani, A.; Hoorfar, M. Microfluidics Integrated Biosensors: A Leading Technology towards Lab-on-a-Chip and Sensing Applications. Sensors 2015, 15, 30011–30031. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.S.; Fan, S.K. Microfluidic Surface Plasmon Resonance Sensors: From Principles to Point-of-Care Applications. Sensors 2016, 16, 1175. [Google Scholar] [CrossRef] [PubMed]
- Dincer, C.; Bruch, R.; Kling, A.; Dittrich, P.S.; Urban, G.A. Multiplexed Point-of-Care Testing-xPOCT. Trends Biotechnol. 2017, 35, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Koczula, K.M.; Gallotta, A. Lateral flow assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; Mabey, D. Point-of-care tests for diagnosing infections in the developing world. Clin. Microbiol. Infect. 2010, 16, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- St John, A.; Price, C.P. Existing and Emerging Technologies for Point-of-Care Testing. Clin. Biochem. Rev. 2014, 35, 155–167. [Google Scholar] [PubMed]
- Posthuma-Trumpie, G.A.; Korf, J.; van Amerongen, A. Lateral flow (immuno) assay: Its strengths, weaknesses, opportunities and threats. A literature survey. Anal. Bioanal. Chem. 2009, 393, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Haushalter, K.J.; Vetcha, S.; Haushalter, R.C. Multiplex Flow Assays. ACS Omega 2016, 1, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Ellington, A.A.; Kullo, I.J.; Bailey, K.R.; Klee, G.G. Antibody-based protein multiplex platforms: Technical and operational challenges. Clin. Chem. 2010, 56, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Supasa, P.; Wongwiwat, W.; Rouvinski, A.; Barba-Spaeth, G.; Duangchinda, T.; Sakuntabhai, A.; Cao-Lormeau, V.M.; Malasit, P.; A Rey, F.; et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat. Immunol. 2016, 17, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Arifin, N.; Riazi, M.; Sadjjadi, S.M.; Muhammad Hafiznur, Y.; Low, H.C.; Zeehaida, M.; Noordin, R. Laboratory detection of strongyloidiasis: IgG-, IgG4—And IgE-ELISAs and cross-reactivity with lymphatic filariasis. Parasite Immunol. 2013, 35, 174–179. [Google Scholar]
- Zhao, Y.; Wang, H.; Zhang, P.; Sun, C.; Wang, X.; Wang, X.; Yang, R.; Wang, C.; Zhou, L. Rapid multiplex detection of 10 foodborne pathogens with an up-converting phosphor technology-based 10-channel lateral flow assay. Sci Rep. 2016, 6, 21342. [Google Scholar] [CrossRef] [PubMed]
- Propper, C.R.; Tester, J.T.; Vail, T.L.; Venkatraman, N. Rapid Multiplex Lateral Flow Assay Device. U.S. Patent 13/869,913, 24 April 2013. [Google Scholar]
- O’Farrell, B. Multiplex Lateral Flow Assays 2015. [PowerPoint]. Available online: http://www.slideshare.net/ofarreb/multiplexing-and-arraying-in-lateral-flow-assays (accessed on 7 September 2017).
- Yen, C.W.; de Puig, H.; Tam, J.O.; Gómez-Márquez, J.; Bosch, I.; Hamad-Schifferli, K.; Gehrke, L. Multicolored silver nanoparticles for multiplexed disease diagnostics: Distinguishing dengue, yellow fever, and Ebola viruses. Lab. Chip. 2015, 15, 1638–1641. [Google Scholar] [CrossRef] [PubMed]
- Corstjens, P.L.A.M.; de Dood, C.J.; van der Ploeg-van Schip, J.J.; Wiesmeijer, K.C.; Riuttamaki, T.; van Meijgaarden, K.E.; Spencer, J.S.; Tanke, H.J.; Ottenhoff, T.H.M.; Geluk, A. Lateral flow assay for simultaneous detection of cellular- and humoral immune responses. Clin. Biochem. 2011, 44, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Seo, H.S.; Kwon, J.H.; Kim, H.T.; Kwon, K.C.; Sim, S.J.; Cha, Y.J.; Lee, J. Multiplex diagnosis of viral infectious diseases (AIDS, hepatitis C, and hepatitis A) based on point of care lateral flow assay using engineered proteinticles. Biosens. Bioelectron. 2015, 69, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Le, T.T.; Chang, P.; Benton, D.J.; McCauley, J.W.; Iqbal, M.; Cass, A.E.G. Dual Recognition Element Lateral Flow Assay Toward Multiplex Strain Specific Influenza Virus Detection. Anal. Chem. 2017, 89, 6781–6786. [Google Scholar] [CrossRef] [PubMed]
- Ch’ng, A.C.W.; Choong, Y.S.; Lim, T.S. Phage Display-Derived Antibodies: Application of Recombinant Antibodies for Diagnostics. In Proof and Concepts in Rapid Diagnostic Tests and Technologies; Saxena, S.K., Ed.; InTech: Rijeka, Croatia, 2016. [Google Scholar]
- Rey, E.G.; O′Dell, D.; Mehta, S.; Erickson, D. Mitigating the Hook Effect in Lateral Flow Sandwich Immunoassays Using Real-Time Reaction Kinetics. Anal. Chem. 2017, 89, 5095–5100. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Hurtado, J.L.; Yetisen, A.K.; Yun, S.H. Multiplex Smartphone Diagnostics. Methods Mol. Biol. 2017, 1546, 295–302. [Google Scholar] [PubMed]
- Guo, T.W.; Laksanasopin, T.; Sridhara, A.A.; Nayak, S.; Sia, S.K. Mobile device for disease diagnosis and data tracking in resource-limited settings. Methods Mol. Biol. 2015, 1256, 3–14. [Google Scholar] [PubMed]
- Laksanasopin, T.; Guo, T.W.; Nayak, S.; Sridhara, A.A.; Xie, S.; Olowookere, O.O.; Cadinu, P.; Meng, F.; Chee, N.H.; Kim, J.; et al. A smartphone dongle for diagnosis of infectious diseases at the point of care. Sci. Transl. Med. 2015, 7, 273re1. [Google Scholar] [CrossRef] [PubMed]
- Bastarache, J.A.; Koyama, T.; Wickersham, N.E.; Ware, L.B. Validation of a multiplex electrochemiluminescent immunoassay platform in human and mouse samples. J. Immunol. Methods 2014, 408, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Bastarache, J.A.; Koyama, T.; Wickersham, N.E.; Mitchell, D.B.; Mernaugh, R.L.; Ware, L.B. Accuracy and reproducibility of a multiplex immunoassay platform: A validation study. J. Immunol. Methods 2011, 367, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Jani, D.; Allinson, J.; Berisha, F.; Cowan, K.J.; Devanarayan, V.; Gleason, C.; Jeromin, A.; Keller, S.; Khan, M.U.; Nowatzke, B.; et al. Recommendations for Use and Fit-for-Purpose Validation of Biomarker Multiplex Ligand Binding Assays in Drug Development. AAPS J. 2016, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]

| Challenge | Difficulty * | Remarks | References (No.) | |
|---|---|---|---|---|
| Technical and Assay Development | Cross-reactivity of immobilized capture and detector antibodies with non-targeted analytes limits multiplexing capability. Antibodies that have been validated on single-assays may be cross-reactive when multiplexed with other assays. Some proteins may not be usable due to nonspecific binding which may reduce assay sensitivity. | Possible | Aptamer oligonucleotides are reportedly cheaper than antibodies. Dual recognition element LFA (DRELFA) has been shown to overcome cross-reactivity of antibody and slow-binding kinetics of aptamers. Phage-display derived recombinant antibodies with potential for higher specificity. Proteinticles (genetically modified proteins) used as 3D probes demonstrate increasing sensitivity of multiplexed LFA for anti-viral detection in HIV, HAV, and HCV. | Lee, et al. [26] Le, et al. [27] Ch’ng, et al. [28] |
| Physical limitation of LFA strip to only a few test lines placed at specific locations, the number of which will affect the test flow rate. | Possible | Pixelation technology for spot array commercially available to test developers since 2015. Parallume lanthanide optical encoding technology enables deep optical multiplexing using a companion reader, proof-of-concept demonstrated for multiplex detection of anti-HIV, anti-HCV etc. Multiple strips incorporated in disc design, allowing detection of 10 different biomarkers simultaneously. | O’Farrell, et al. [15] Haushalter, et al. [17] Zhao, et al. [21] | |
| Clinical specimens for assay development and test validation will require patients with multiple co-infections relevant to the test, which may be difficult to acquire. | Difficult-Possible | Available cohort studies monitoring and diagnosing several diseases relevant to the local epidemiology may be an important source of specimens with relevant co-infections. | ||
| Hook effect arising from an excess of unlabeled analytes competing with labeled analytes causes decrease in test signal for samples with high analyte concentration. Compounded in multiplexed assays with cross-reactivity. | Possible | With the use of a portable imaging devices, a reaction kinetics-based technique (example: C-reactive protein) has been proposed to significantly increase the dynamic range of LFAs, overcoming the problem of hook effect. | Rey, et al. [29] | |
| Operational and Quality Control | Inter- and intra-assay variability (estimated by coefficient of variation (CV)) for multiplexed test are questionable and acceptable values of reproducibility in multiplexed tests undefined. | Difficult | Limited guidance/regulation available for development of multiplexed LFAs may require more concerted effort from authoritative diagnostic and regulatory bodies. | Ellington, et al. 2010 [18] |
| Acceptable level of imprecision of LF assays undefined. Unclear whether failure of one test within the multiplex constitutes entire test failure. | ||||
| Conventional LFAs with visual interpretation become complicated with increasing number of test results and corresponding controls, particularly with positive results with low signals. | Possible | Test results may be varied using different labels (e.g., multicolored silver particles), or structures/shapes. Portable battery-operated readers, smartphones with diagnostic applications or accessories (dongles) are becoming readily available to remove reliance on user-interpretation, and allows for alternative probes and also produce data for analytical and monitoring. | Yen, et. al. [24] Martinez-Hurtado, et. al., [30] Guo, et al. [31] Laksanasopin, et al. [32] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Hanafiah, K.; Arifin, N.; Bustami, Y.; Noordin, R.; Garcia, M.; Anderson, D. Development of Multiplexed Infectious Disease Lateral Flow Assays: Challenges and Opportunities. Diagnostics 2017, 7, 51. https://doi.org/10.3390/diagnostics7030051
Mohd Hanafiah K, Arifin N, Bustami Y, Noordin R, Garcia M, Anderson D. Development of Multiplexed Infectious Disease Lateral Flow Assays: Challenges and Opportunities. Diagnostics. 2017; 7(3):51. https://doi.org/10.3390/diagnostics7030051
Chicago/Turabian StyleMohd Hanafiah, Khayriyyah, Norsyahida Arifin, Yazmin Bustami, Rahmah Noordin, Mary Garcia, and David Anderson. 2017. "Development of Multiplexed Infectious Disease Lateral Flow Assays: Challenges and Opportunities" Diagnostics 7, no. 3: 51. https://doi.org/10.3390/diagnostics7030051
APA StyleMohd Hanafiah, K., Arifin, N., Bustami, Y., Noordin, R., Garcia, M., & Anderson, D. (2017). Development of Multiplexed Infectious Disease Lateral Flow Assays: Challenges and Opportunities. Diagnostics, 7(3), 51. https://doi.org/10.3390/diagnostics7030051