Visual Detection of Dengue-1 RNA Using Gold Nanoparticle-Based Lateral Flow Biosensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Oligonucleotides
2.3. Preparation of AuNP and AuNP/rDNA Conjugate
2.4. Immobilization of dDNA and cDNA Probes
2.5. Assembly of LFB
2.6. Assay Protocol
2.7. Quantitation of LFB Signal
2.8. DENV-1 Strain
2.9. Extraction and Amplification of DENV-1 RNA
3. Results and Discussion
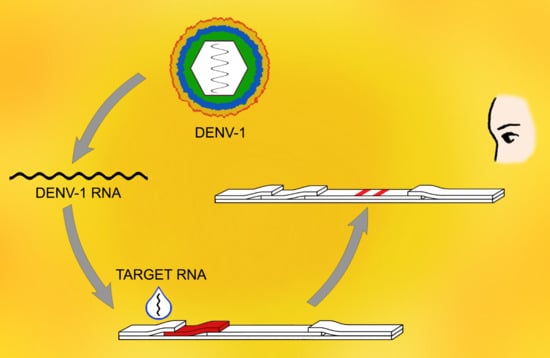
3.1. Principle of Detection
3.2. Optimization of Experimental Conditions
3.3. Analytical Characteristics
3.4. Selectivity Tests
3.5. Detection of Dengue-1 Viral RNA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sim, S.; Hibberd, M.L. Genomic approaches for understanding dengue: Insights from the virus, vector, and host. Genome Biol. 2016, 1, 738. [Google Scholar] [CrossRef] [PubMed]
- Villabona-Arenas, C.J.; Zanotto, P.M. Worldwide Spread of Dengue Virus Type 1. PLoS ONE 2013, 8, e62649. [Google Scholar] [CrossRef] [PubMed]
- Bäck, A.; Lundkvist, Å. Dengue viruses—An overview. Infect. Ecol. Epidemiol. 2013, 3, 19839. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Falgout, B.; Markoff, L. Identification of specific nucleotide sequences within the conserved 3’-SL in the dengue type 2 virus genome required for replication. J. Virol. 1998, 72, 7510–7522. [Google Scholar] [PubMed]
- Balmaseda, A.; Hammond, S.N.; Pérez, L.; Tellez, Y.; Saborío, S.I.; Mercado, J.C.; Harris, E. Serotype-specific differences in clinical manifestations of dengue. Am. J. Trop. Med. Hyg. 2006, 74, 449–456. [Google Scholar] [CrossRef]
- Parkash, O.; Shueb, R. Diagnosis of Dengue Infection Using Conventional and Biosensor Based Techniques. Viruses 2015, 7, 5410–5427. [Google Scholar] [CrossRef]
- Baeumner, A.J.; Schlesinger, N.A.; Slutzki, N.S.; Romano, J.; Lee, E.M.; Montagna, R.A. Biosensor for Dengue Virus Detection: Sensitive, Rapid, and Serotype Specific. Anal. Chem. 2002, 74, 1442–1448. [Google Scholar] [CrossRef]
- Blacksell, S.D.; Mammen, M.P.; Thongpaseuth, S.; Gibbons, R.V.; Jarman, R.G.; Jenjaroen, K.; Day, N.P.J. Evaluation of the Panbio dengue virus nonstructural 1 antigen detection and immunoglobulin M antibody enzyme-linked immunosorbent assays for the diagnosis of acute dengue infections in Laos. Diagn. Microbiol. Infect. Dis. 2008, 60, 43–49. [Google Scholar] [CrossRef]
- Darwish, N.T.; Sekaran, S.D.; Khor, S.M. Point-of-care tests: A review of advances in the emerging diagnostic tools for dengue virus infection. Sens. Actuators B Chem 2018, 255, 3316–3331. [Google Scholar] [CrossRef]
- Balaji, A.; Yang, S.; Wang, J.; Zhang, J. Graphene oxide-based nanostructured DNA ensor. Biosensors 2019, 9, 74. [Google Scholar] [CrossRef]
- Carter, D.J.; Cary, R.B. Lateral flow microarrays: A novel platform for rapid nucleic acid detection based on miniaturized lateral flow chromatography. Nucleic Acids Res. 2007, 35, e74. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Macdonald, J. Multiplexed lateral flow biosensors: Technological advances for radically improving point-of-care diagnoses. Biosens. Bioelectron. 2016, 85, 998–999. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xu, L.-P.; Zhou, S.-F.; Liu, G.; Zhang, X. Recent Advances in Nanoparticles-based Lateral Flow Biosensors. Am. J. Med. Sci. 2014, 6, 41–57. [Google Scholar] [CrossRef]
- Sajid, M.; Kawde, A.N.; Daud, M. Designs, formats and applications of lateral flow assay: A literature review. J. Saudi Chem. Soc. 2015, 19, 689–705. [Google Scholar] [CrossRef] [Green Version]
- Quesada-González, D.; Merkoçi, A. Nanoparticle-based lateral flow biosensors. Biosens. Bioelectron. 2015, 73, 47–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, G.; Zhang, S.; Hu, X. Signal enhancement in a lateral flow immunoassay based on dual gold nanoparticle conjugates. Clin. Biochem. 2013, 46, 1734–1738. [Google Scholar] [CrossRef]
- Sekaran, S.D.; Soe, H.J. Issues in contemporary and potential future molecular diagnostics for dengue. Expert Rev. Mol. Diagn 2017, 17, 217–223. [Google Scholar] [CrossRef]
- Wu, S.L.; Lee, E.U.N.M.I.; Putvatana, R.; Shurtliff, R.N.; Porter, K.R.; Suharyono, W.; Romano, J.W. Detection of Dengue Viral RNA Using a Nucleic Acid Sequence-Based Amplification Assay. J. Clin. Microbiol. 2001, 39, 2794–2798. [Google Scholar] [CrossRef] [Green Version]
- Anderson, M.J.; Torres-Chavolla, E.; Castro, B.A.; Alocilja, E.C. One step alkaline synthesis of biocompatible gold nanoparticles using dextrin as capping agent. J. Nanoparticle Res. 2011, 13, 2843–2851. [Google Scholar] [CrossRef]
- Hill, H.D.; Mirkin, C.A. The bio-barcode assay for the detection of protein and nucleic acid targets using DTT-induced ligand exchange. Nat. Protoc. 2006, 1, 324–336. [Google Scholar] [CrossRef]
- Gao, X.; Xu, H.; Baloda, M.; Gurung, A.S.; Xu, L.-P.; Wang, T.; Liu, G. Visual detection of microRNA with lateral flow nucleic acid biosensor. Biosens. Bioelectron. 2014, 54, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.V.; Dantzler, J.L.; Weigl, B.H. Analytical Tools to Improve Optimization Procedures for Lateral Flow Assays. Diagnostics 2017, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Ma, Y.; Zhang, A.; Zhang, L.; Zeng, L.; Liu, G. Disposable Nucleic Acid Biosensors Based on Gold Nanoparticle Probes and Lateral Flow Strip. Anal. Chem. 2009, 81, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lang, H.P.; Yoshikawa, G.; Gerber, C. Optimization of DNA hybridization efficiency by pH-driven nanomechanical bending. Langmuir 2012, 28, 6494–6501. [Google Scholar] [CrossRef] [PubMed]
- Koczula, K.M.; Gallotta, A. Lateral flow assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y. Secondary structure effects on DNA hybridization kinetics: A solution versus surface comparison. Nucleic Acids Res. 2006, 34, 3370–3377. [Google Scholar] [CrossRef] [PubMed]
- Berndt, A.; Kosmehl, H.; Celeda, D.; Katenkamp, D. Reduced formamide content and hybridization temperature results in increased non-radioactive mRNA in situ hybridization signals. Acta Histochem. 1996, 98, 79–87. [Google Scholar] [CrossRef]
- Matthiesen, S.H.; Hansen, C.M. Fast and Non-Toxic In situ Hybridization without Blocking of Repetitive Sequences. PLoS ONE 2012, 7, e40675. [Google Scholar] [CrossRef]
- Fuchs, J.; Dell’Atti, D.; Buhot, A.; Calemczuk, R.; Mascini, M.; Livache, T. Effects of formamide on the thermal stability of DNA duplexes on biochips. Anal. Biochem. 2010, 397, 132–134. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Wang, M.Z.; Chen, Z.L.; Fang, J.H.; Fang, M.M.; Liu, J.; Yu, X.P. Development of a colloidal gold-based lateral-flow immunoassay for the rapid simultaneous detection of clenbuterol and ractopamine in swine urine. Anal. Bioanal. Chem. 2009, 395, 2591–2599. [Google Scholar] [CrossRef]
- Staples, J.E.; Breiman, R.F.; Powers, A.M. Chikungunya Fever: An Epidemiological Review of a Re-Emerging Infectious Disease. Clin. Infect. Dis. 2009, 49, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Colón-González, F.J.; Peres, C.A.; Steiner São Bernardo, C.; Hunter, P.R.; Lake, I.R. After the epidemic: Zika virus projections for Latin America and the Caribbean. PLOS Negl. Trop. Dis. 2017, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Göertz, G.P.; Vogels, C.B.F.; Geertsema, C.; Koenraadt, C.J.M.; Pijlman, G.P. Mosquito co-infection with Zika and chikungunya virus allows simultaneous transmission without affecting vector competence of Aedes aegypti. PLOS Negl. Trop. Dis. 2017, 11, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Faye, O.; Faye, O.; Diallo, D.; Diallo, M.; Weidmann, M.; Sall, A.A. Quantitative real-time PCR detection of Zika virus and evaluation with field-caught Mosquitoes. Virol. J. 2013, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Yeung, C.Y.; Chen, P.H.; Yeh, M.K.; Hou, S.Y. Salmonella detection using 16S ribosomal DNA/RNA probe-gold nanoparticles and lateral flow immunoassay. Food Chem. 2013, 141, 2526–2532. [Google Scholar] [CrossRef] [PubMed]
- Anfossi, L.; Nardo, F.; Di Giovannoli, C.; Passini, C. Increased sensitivity of lateral flow immunoassay for ochratoxin A through silver enhancement. Anal. Bioanal Chem. 2013, 405, 9859–9867. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Oligonucleotide | Sequence |
|---|---|
| DENV-1 probe | 5′-GGGAAGCTGTATCCTGGTGGTAAGG(A)14-3′-biotin |
| Reporter probe | Thiol-5′-GATGCAAGGTCGCATATGAG-3′ |
| Control probe | Biotin-5′-CTCATATGCGACCTTGCATC-3′ |
| Primer 1 [a] | 5′-aattctaatacgactcactatagggGAGACAGCAGGATCTCTGGTCT-3′ |
| Primer 2 [b] | 5′-gatgcaaggtcgcatatgagGGTTAGAGGAGACCCCTCCC-3′ |
| Sample | Concentration | LFB result (+/−) |
|---|---|---|
| (−) LFB control | 0 | (−) |
| (−) Extraction control | 0 | (−) |
| (−) NASBA control | 0 | (−) |
| DENV-1 | 1.2 × 102 pfu/mL | (−) |
| DENV-1 | 1.2 × 103 pfu/mL | (−) |
| DENV-1 | 1.2 × 104 pfu/mL | (+) |
| T-RNA | 0.1 µM | (+) |
| T-1 | 0.02–0.08 µM | All (+) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yrad, F.M.; Castañares, J.M.; Alocilja, E.C. Visual Detection of Dengue-1 RNA Using Gold Nanoparticle-Based Lateral Flow Biosensor. Diagnostics 2019, 9, 74. https://doi.org/10.3390/diagnostics9030074
Yrad FM, Castañares JM, Alocilja EC. Visual Detection of Dengue-1 RNA Using Gold Nanoparticle-Based Lateral Flow Biosensor. Diagnostics. 2019; 9(3):74. https://doi.org/10.3390/diagnostics9030074
Chicago/Turabian StyleYrad, Flora M., Josephine M. Castañares, and Evangelyn C. Alocilja. 2019. "Visual Detection of Dengue-1 RNA Using Gold Nanoparticle-Based Lateral Flow Biosensor" Diagnostics 9, no. 3: 74. https://doi.org/10.3390/diagnostics9030074
APA StyleYrad, F. M., Castañares, J. M., & Alocilja, E. C. (2019). Visual Detection of Dengue-1 RNA Using Gold Nanoparticle-Based Lateral Flow Biosensor. Diagnostics, 9(3), 74. https://doi.org/10.3390/diagnostics9030074