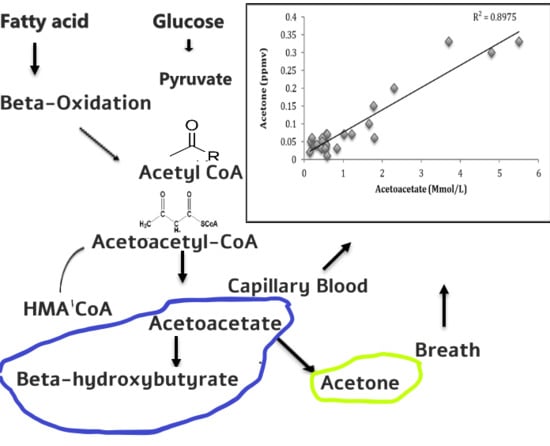
Blood Ketone Bodies and Breath Acetone Analysis and Their Correlations in Type 2 Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Collection of Samples
2.3. Biochemical Analysis in the Blood
2.4. Breath Acetone Analysis Using HS-SPME/GC-MS
2.5. Statistical Analysis
3. Results
3.1. Biochemical Analysis
3.2. Breath Acetone Analysis Using HS-SPME/GC-MS
3.3. Correlation Studies of Breath Acetone and Blood Ketone Bodies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yamada, K.; Ohishi, K.; Gilbert, A.; Akasaka, M.; Yoshida, N.; Yoshimura, R. Measurement of natural carbon isotopic composition of acetone in human urine. Anal. Bioanal. Chem. 2016, 408, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.; Porto-Figueira, P.; Cavaco, C.; Taunk, K.; Rapole, S.; Dhakne, R.; Nagarajaram, H.; Cmara, J.S. Breath analysis as a potential and non-invasive frontier in disease diagnosis: An overview. Metabolites 2015, 5, 3–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, M.; Wang, Z.; Yuan, Y.; Chen, Z.; Zhao, X. Continuous Monitoring of Breath Acetone, Blood Glucose and Blood Ketone in 20 Type 1 Diabetic Outpatients Over 30 Days. J. Anal. Bioanal. Tech. 2017, 8, 2155–9872. [Google Scholar] [CrossRef] [Green Version]
- Newton, C.A.; Raskin, P. Diabetic ketoacidosis in type 1 and type 2 diabetes mellitus: Clinical and biochemical differences. Arch. Intern. Med. 2004, 164, 1925–1931. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Reyes, A.; Horsten, R.C.; Urbach, H.P.; Bhattacharya, N. Study of the exhaled acetone in type 1 diabetes using quantum cascade laser spectroscopy. Anal. Chem. 2014, 87, 507–512. [Google Scholar] [CrossRef]
- Tassopoulos, C.N.; Barnett, D.; Fraser, T.R. Breath-acetone and blood-sugar measurements in diabetes. Lancet 1969, 293, 1282–1286. [Google Scholar] [CrossRef]
- Turner, C.; Walton, C.; Hoashi, S.; Evans, M. Breath acetone concentration decreases with blood glucose concentration in type I diabetes mellitus patients during hypoglycaemic clamps. J. Breath Res. 2009, 3, 046004. [Google Scholar] [CrossRef]
- Anderson, J.C. Measuring breath acetone for monitoring fat loss. Obesity 2015, 23, 2327–2334. [Google Scholar] [CrossRef]
- Prabhakar, A.; Quach, A.; Wang, D.; Zhang, H.; Terrera, M.; Jackemeyer, D.; Xian, X.; Tsow, F.; Tao, N.; Forzanil, E. Breath acetone as biomarker for lipid oxidation and early ketone detection. Glob. J. Obes. Diabetes Metab. Syndr. 2014, 1, 12. [Google Scholar]
- Laffel, L. Ketone bodies: A review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab. Res. 1999, 15, 412–426. [Google Scholar] [CrossRef]
- Henderson, M.J.; Karger, B.; Wrenshall, G. Acetone in the breath. Diabetes 1952, 1, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Mbi, A.; Shepherd, M. A study on breath acetone in diabetic patients using a cavity ringdown breath analyzer: Exploring correlations of breath acetone with blood glucose and glycohemoglobin A1C. IEEE Sens. J. 2010, 10, 54–63. [Google Scholar] [CrossRef]
- Fan, G.; Yang, C.; Lin, C.; Chen, C.; Shih, C. Applications of Hadamard transform-gas chromatography/mass spectrometry to the detection of acetone in healthy human and diabetes mellitus patient breath. Talanta 2014, 120, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Zhang, J.; Yu, X.; Zhang, W.; Zhang, X. Determination of acetone in human breath by gas chromatography–mass spectrometry and solid-phase microextraction with on-fiber derivatization. J. Chromatogr. B 2004, 810, 269–275. [Google Scholar] [CrossRef]
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef]
- Motala, Y.A.; Fraser, M.A.K.; Pirie, J. Epidemiology of Type 1 and Type 2 Diabetes in Africa. Eur. J. Prevent. Cardiol. 2003, 10, 77–83. [Google Scholar] [CrossRef]
- Bello-Sani, F.; Bakari, A.G.; Anumah, F.E. Dyslipidaemia in persons with type 2 diabetes mellitus in Kaduna, Nigeria. Int. J. Diabetes Metab. 2007, 15, 9. [Google Scholar] [CrossRef] [Green Version]
- Saasa, V.; Malwela, T.; Beukes, M.; Mokgotho, M.; Liu, C.; Mwakikunga, B. Sensing Technologies for Detection of Acetone in Human Breath for Diabetes Diagnosis and Monitoring. Diagnostics 2018, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Blaikie, T.P.; Edge, J.A.; Hancock, G.; Lunn, D.; Megson, C.; Peverall, R.; Richmond, G.; Ritchie, G.A.; Taylor, D. Comparison of breath gases, including acetone, with blood glucose and blood ketones in children and adolescents with type 1 diabetes. J. Breath Res. 2014, 8, 046010. [Google Scholar] [CrossRef]
- Stephens, J.M.; Sulway, M.J.; Watkins, P.J. Relationship of blood acetoacetate and 3-hydroxybutyrate in diabetes. Diabetes 1971, 20, 485–489. [Google Scholar] [CrossRef]
- Newsholme, P.; Curi, R.; Gordon, S.; Newsholme, E.A. Metabolism of glucose, glutamine, long-chain fatty acids and ketone bodies by murine macrophages. Biochem. J. 1986, 239, 121. [Google Scholar] [CrossRef] [PubMed]
- Minh, T.D.; Oliver, S.R.; Ngo, J.; Flores, R.; Midyett, J.; Meinardi, S.; Carlson, M.K.; Rowland, F.S.; Blake, D.R.; Galassetti, P.R. Noninvasive measurement of plasma glucose from exhaled breath in healthy and type 1 diabetic subjects. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E1166–E1175. [Google Scholar] [CrossRef] [Green Version]
- Novak, B.J.; Blake, D.R.; Meinardi, S.; Rowland, F.S.; Pontello, A.; Cooper, D.M.; Galassetti, P.R. Exhaled methyl nitrate as a noninvasive marker of hyperglycemia in type 1 diabetes. Proc. Natl. Acad. Sci. USA 2007, 104, 15613–15618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, A.W.; Sagarduy, A.; Ericsson, E.; Arnqvist, H.J. Concentrations of acetone in venous blood samples from drunk drivers, type-I diabetic outpatients, and healthy blood donors. J. Anal. Toxicol. 1993, 17, 182–185. [Google Scholar] [CrossRef]
- Klocker, A.A.; Phelan, H.; Twigg, S.M.; Craig, M.E. Blood β-hydroxybutyrate vs. urine acetoacetate testing for the prevention and management of ketoacidosis in Type 1 diabetes: A systematic review. Diabetic Med. 2013, 30, 818–824. [Google Scholar] [CrossRef]
- Balasse, E.O.; Féry, F. Ketone body production and disposal: Effects of fasting, diabetes, and exercise. Diabetes Metab. 1989, 5, 247–270. [Google Scholar] [CrossRef]
- Yamane, N.; Tsuda, T.; Nose, K.; Yamamoto, A.; Ishiguro, H.; Kondo, T. Relationship between skin acetone and blood β-hydroxybutyrate concentrations in diabetes. Clin. Chim. Acta 2006, 365, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Taboulet, P.; Deconinck, N.; Thurel, A.; Haas, L.; Manamani, J.; Porcher, R.; Schmit, C.; Fontaine, J.; Gautier, J. Correlation between urine ketones (acetoacetate) and capillary blood ketones (3-beta-hydroxybutyrate) in hyperglycaemic patients. Diabetes Metab. 2007, 33, 135–139. [Google Scholar] [CrossRef]
- Byrne, H.A.; Tieszen, K.L.; Hollis, S.; Dornan, T.L.; New, J.P. Evaluation of an electrochemical sensor for measuring blood ketones. Diabetes Care 2000, 23, 500–503. [Google Scholar] [CrossRef] [Green Version]
- Osuna, E.; Vivero, G.; Conejero, J.; Abenza, J.M.; Martínez, P.; Luna, A.; Pérez-Cárceles, M.D. Postmortem vitreous humor β-hydroxybutyrate: Its utility for the postmortem interpretation of diabetes mellitus. Forensic Sci. Int. 2005, 153, 189–195. [Google Scholar] [CrossRef]
- Kanikarla-Marie, P.; Jain, S.K. Hyperketonemia and ketosis increase the risk of complications in type 1 diabetes. Free Radic. Biol. Med. 2016, 95, 268–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasution, T.I.; Nainggolan, I.; Hutagalung, S.D.; Ahmad, K.R.; Ahmad, Z.A. The sensing mechanism and detection of low concentration acetone using chitosan-based sensors. Sens. Actuat. B Chem. 2013, 177, 522–528. [Google Scholar] [CrossRef]
- Wang, L.; Kalyanasundaram, K.; Stanacevic, M.; Gouma, P. Nanosensor device for breath acetone detection. Sens. Lett. 2010, 8, 709–712. [Google Scholar] [CrossRef]
- Xiao, T.; Wang, X.; Zhao, Z.; Li, L.; Zhang, L.; Yao, H.; Wang, J.; Li, Z. Highly sensitive and selective acetone sensor based on C-doped WO3 for potential diagnosis of diabetes mellitus. Sens. Actuat. B Chem. 2014, 199, 210–219. [Google Scholar] [CrossRef]





| Biochemical Parameters | Type 2 DM (n = 30) | Non-Diabetes (n = 30) | p-Value |
|---|---|---|---|
| Age | 47 ± 10 | 41 ± 10 | <0.001 |
| Gender | 13/17 | 11/19 | 0.10 |
| BMI (kg·m−2) | 28.4 ± 4.5 | 25.4 ± 4.0 | 0.47 |
| Plasma glucose (mmol/L) | 8.6 ± 2.43 | 5.7 ± 1.44 | 0.007 |
| HB1Ac (%) | 10.3 ± 2.57 | - | - |
| Total cholesterol (mmol/L) | 5.10 ± 1.40 | 4.5 ± 1.42 | 0.17 |
| Triglycerides (mmol/L) | 1.57 ± 1.3 | 1.04 ± 1 | 0.01 |
| HDL cholesterol (mmol/L) | 1.15 ± 0.27 | 1.33 ± 0.47 | 0.34 |
| LDL cholesterol (mmol/L) | 2.56 ± 1.32 | 2.43 ± 0.97 | 0.52 |
| Β-hydroxybutyrate (mmol/L) | 0.46 ± 0.02 | 0.44 ± 0.41 | 0.55 |
| Acetoacetate (mmol/L) | 0.09 ± 0.02 | 0.05 ± 0.03 | 0.47 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saasa, V.; Beukes, M.; Lemmer, Y.; Mwakikunga, B. Blood Ketone Bodies and Breath Acetone Analysis and Their Correlations in Type 2 Diabetes Mellitus. Diagnostics 2019, 9, 224. https://doi.org/10.3390/diagnostics9040224
Saasa V, Beukes M, Lemmer Y, Mwakikunga B. Blood Ketone Bodies and Breath Acetone Analysis and Their Correlations in Type 2 Diabetes Mellitus. Diagnostics. 2019; 9(4):224. https://doi.org/10.3390/diagnostics9040224
Chicago/Turabian StyleSaasa, Valentine, Mervyn Beukes, Yolandy Lemmer, and Bonex Mwakikunga. 2019. "Blood Ketone Bodies and Breath Acetone Analysis and Their Correlations in Type 2 Diabetes Mellitus" Diagnostics 9, no. 4: 224. https://doi.org/10.3390/diagnostics9040224
APA StyleSaasa, V., Beukes, M., Lemmer, Y., & Mwakikunga, B. (2019). Blood Ketone Bodies and Breath Acetone Analysis and Their Correlations in Type 2 Diabetes Mellitus. Diagnostics, 9(4), 224. https://doi.org/10.3390/diagnostics9040224