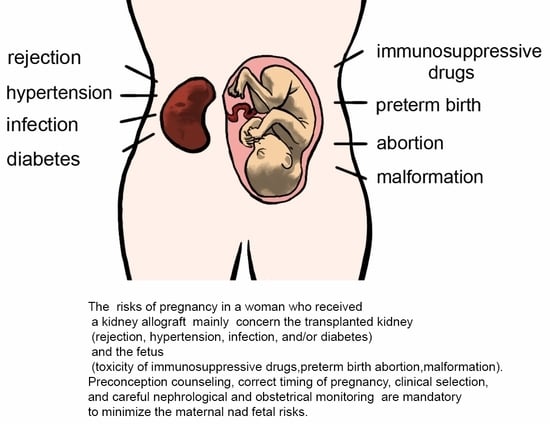
Planned Pregnancy in Kidney Transplantation. A Calculated Risk
Abstract
:1. Introduction
2. Planning Posttransplant Pregnancy
3. Maternal Medical Management and Outcomes during Pregnancy
3.1. Infection
3.2. Hypertension
3.3. Preeclampsia
3.4. Thrombotic Microangiopathy
3.5. Kidney Dysfunction
3.6. Diabetes Mellitus
4. Immunosuppressive Drugs and Fetal Outcome
4.1. Relatively Safe Drugs
4.2. Drugs with Uncertain Safety
4.3. Drugs with Fetal Toxicity
4.4. Vaginal or Cesarean Delivery
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pezeshki, M.; Taherian, A.A.; Gharavy, M.; Ledger, W.L. Menstrual characteristics and pregnancy in women after renal transplantation. Int. J. Gynaecol. Obstet. 2004, 85, 119–125. [Google Scholar] [CrossRef]
- Rao, S.; Ghanta, M.; Moritz, M.J.; Constantinescu, S. Long-Term Functional Recovery, Quality of Life, and Pregnancy After Solid Organ Transplantation. Med. Clin. N. Am. 2016, 100, 613–629. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.; Bramham, K.; Moritz, M.J.; Coscia, L. Reproductive health in women following abdominal organ transplant. Am. J. Transpl. 2018, 18, 1068–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piccoli, G.B.; Cabiddu, G.; Attini, R.; Gerbino, M.; Todeschini, P.; Perrino, M.L.; Manzione, A.M.; Piredda, G.B.; Gnappi, E.; Caputo, F.; et al. Outcomes of Pregnancies After Kidney Transplantation: Lessons Learned From CKD. A Comparison of Transplanted, Nontransplanted Chronic Kidney Disease Patients and Low-Risk Pregnancies: A Multicenter Nationwide Analysis. Transplantation 2017, 101, 2536–2544. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Venkatesan, R.L.; Gupta, A.; Sanghavi, M.K.; Welge, J.; Johansen, R.; Kean, E.B.; Kaur, T.; Gupta, A.; Grant, T.J.; et al. Pregnancy outcomes in women with kidney transplant: Metaanalysis and systematic review. BMC Nephrol. 2019, 20, 24. [Google Scholar] [CrossRef] [PubMed]
- Davison, J.M.; Baylis, C. Pregnancy in patients with underlying renal disease. In Oxford Textbook of Clinical Nephrology; Davison, A.M., Cameron, J.S., Grunfeld, J.P., Ponticelli, C., Ritz, E., Winearls, C.G., van Ypersele, C., Eds.; Oxford University Press: Oxford, UK, 2005; Volume 3, pp. 2243–2259. [Google Scholar]
- McKay, D.B.; Josephson, M.A.; Armenti, V.T.; August, P.; Coscia, L.A.; Davis, C.L.; Davison, J.M.; Easterling, T.; Friedman, J.E.; Hou, S.; et al. Reproduction and transplantation: Report on the AST Consensus Conference on Reproductive Issues and Transplantation. Am. J. Transpl. 2005, 5, 1592–1599. [Google Scholar] [CrossRef] [Green Version]
- Coscia, L.A.; Constantinescu, S.; Moritz, M.J.; Frank, A.M.; Ramirez, C.B.; Maley, W.R.; Doria, C.; McGrory, C.H.; Armenti, V.T. Report from the National Transplantation Pregnancy Registry (NTPR): Outcomes of pregnancy after transplantation. Clin. Transpl. 2010, 65–85. [Google Scholar]
- Scheres, L.J.J.; Lijfering, W.M.; Groenewegen, N.F.M.; Koole, S.; de Groot, C.J.M.; Middeldorp, S.; Cannegieter, S.C. Hypertensive Complications of Pregnancy and Risk of Venous Thromboembolism. Hypertension 2020, 75, 781–787. [Google Scholar] [CrossRef]
- Stegeman, B.H.; de Bastos, M.; Rosendaal, F.R.; van Hylckama Vlieg, A.; Helmerhorst, F.M.; Stijnen, T.; Dekkers, O.M. Different combined oral contraceptives and the risk of venous thrombosis: Systematic review and network meta-analysis. BMJ 2013, 347, 5298. [Google Scholar] [CrossRef] [Green Version]
- Paulen, M.E.; Folger, S.G.; Curtis, K.M.; Jamieson, D.J. Contraceptive use among solid organ transplant patients: A systematic review. Contraception 2010, 82, 102–112. [Google Scholar] [CrossRef]
- Yousif, M.E.; Bridson, J.M.; Halawa, A. Contraception After Kidney Transplantation, From Myth to Reality: A Comprehensive Review of the Current Evidence. Exp. Clin. Transpl. 2016, 14, 252–258. [Google Scholar]
- Huguelet, P.S.; Sheehan, C.; Spitzer, R.F.; Scott, S. Use of the levonorgestrel 52-mg intrauterine system in adolescent and young adult solid organ transplant recipients: A case series. Contraception 2017, 95, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Midtvedt, K.; Asberg, A.; Eide, I.A. Immunosuppression and Reproductive Health After Kidney Transplantation. Transplantation 2019, 103, e325–e333. [Google Scholar] [CrossRef] [PubMed]
- Attini, R.; Cabiddu, G.; Montersino, B.; Gammaro, L.; Gernone, G.; Moroni, G.; Santoro, D.; Spotti, D.; Masturzo, B.; Gazzani, I.B.; et al. Contraception in chronic kidney disease: A best practice position statement by the Kidney and Pregnancy Group of the Italian Society of Nephrology. J. Nephrol. 2020, 33, 1343–1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKay, D.B.; Josephson, M.A. Pregnancy after transplantation. Clin. J. Am. Soc. Nephrol. 2008, 3 (Suppl. 2), S117–S125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, C.; Gill, J.; Zalunardo, N.; Johnston, O.; Mehrotra, A.; Gill, J.S. Timing of pregnancy after kidney transplantation and risk of allograft failure. Am. J. Transpl. 2016, 16, 2360–2367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EBPG Expert Group on Renal Transplantation. European best practice guidelines for renal transplantation. Section IV: Long-term management of the transplant recipient. IV.10. Pregnancy in renal transplant recipients. Nephrol. Dial. Transpl. 2002, 17 (Suppl. 4), 50–55. [Google Scholar]
- Davison, J.M.; Bailey, D.J. Pregnancy after transplantation. J. Obstet. Gynaecol. Res. 2003, 29, 227–233. [Google Scholar] [CrossRef]
- Cabiddu, G.; Spotti, D.; Gernone, G.; Santoro, D.; Moroni, G.; Gregorini, G.; Giacchino, F.; Attini, R.; Limardo, M.; Gammaro, L.; et al. A best-practice position statement on pregnancy after kidney transplantation: Focusing on the unsolved questions. The Kidney and Pregnancy Study Group of the Italian Society of Nephrology. J. Nephrol. 2018, 31, 665–681. [Google Scholar] [CrossRef] [Green Version]
- Danziger-Isakov, L.; Kumar, D. AST ID Community of Practice. Vaccination of solid organ transplant candidates and recipients: Guidelines from the American society of transplantation infectious diseases community of practice. Clin. Transpl. 2019, 33, e13563. [Google Scholar] [CrossRef]
- Zhu, L.; Gong, N.; Liu, B.; Lu, X.; Chen, D.; Chen, S.; Shu, H.; Ma, K.; Xu, X.; Guo, Z.; et al. Coronavirus Disease 2019 pneumonia in immunosuppressed renal transplant recipients: A summary of 10 confirmed cases in Wuhan China. Eur. Urol. 2020, 77, 748–754. [Google Scholar] [CrossRef]
- Banerjee, D.; Popoola, J.; Shah, S.; Ster, I.C.; Quan, V.; Phanish, M. COVID-19 infection in kidney transplant recipients. Kidney Int. 2020, 97, 1076–1082. [Google Scholar] [CrossRef]
- Alberici, F.; Delbarba, E.; Manenti, E.; Econimo, L.; Valerio, F.; Pola, A.; Maffei, C.; Possenti, S.; Zambetti, N.; Moscato, M.; et al. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020, 97, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Caillard, S.; Chavarot, N.; Francois, H.; Matignon, M.; Greze, C.; Kamar, N.; Gatault, P.; Thaunat, O.; Legris, T.; Frimat, L.; et al. Is COVID-19 infection more severe in kidney transplant recipients? Am. J. Transpl. 2021, 21, 1295–1303. [Google Scholar] [CrossRef]
- Tillett, R.L.; Sevinsky, J.R.; Hartley, P.D.; Kerwin, H.; Crawford, N.; Gorzalski, A.; Laverdure, C.; Verma, S.C.; Rossetto, C.C.; Jackson, D.; et al. Genomic evidence for reinfection with SARS-CoV-2: A case study. Lancet Infect. Dis. 2021, 21, 52–58. [Google Scholar] [CrossRef]
- Piccoli, G.B.; Cabiddu, G.; Attini, R.; Vigotti, F.N.; Maxia, S.; Lepori, N.; Tuveri, M.; Massidda, M.; Marchi, C.; Mura, S.; et al. Risk of Adverse Pregnancy Outcomes in Women with CKD. J. Am. Soc. Nephrol. 2015, 26, 2011–2022. [Google Scholar] [CrossRef] [Green Version]
- Mor, G.; Cardenas, I. The immune system in pregnancy: A unique complexity. Am. J. Reprod. Immunol. 2010, 63, 425–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Souza, R.M.; Olsburgh, J. Urinary tract infection in the renal transplant patient. Nat. Clin. Pract. Nephrol. 2008, 4, 252–264. [Google Scholar] [CrossRef]
- Yan, L.; Jin, Y.; Hang, H.; Yan, B. The association between urinary tract infection during pregnancy and preeclampsia: A meta-analysis. Medicine 2018, 97, e12192. [Google Scholar] [CrossRef]
- Leruez-Ville, M.; Foulon, I.; Pass, R.; Ville, Y. Cytomegalovirus infection during pregnancy: State of the science. Am. J. Obstet. Gynecol. 2020, 223, 330–349. [Google Scholar] [CrossRef] [PubMed]
- Ponticelli, C.; Cucchiari, D.; Graziani, G. Hypertension in kidney transplant recipients. Transpl. Int. 2011, 24, 523–533. [Google Scholar] [CrossRef]
- Rubin, M.F. Hypertension following kidney transplantation. Adv. Chronic Kidney Dis. 2011, 18, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Mastrobattista, J.M.; Gomez-Lobo, V. Society for Maternal-Fetal Medicine, authors. Pregnancy after solid organ transplantation. Obstet. Gynecol. 2008, 112, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, N.A.; James, N.T.; Kucirka, L.M.; Boyarsky, B.J.; Garonzik-Wang, J.M.; Montgomery, R.A.; Segev, D.L. Pregnancy outcomes in kidney transplant recipients: A systematic review and meta-analysis. Am. J. Transplant. 2011, 11, 2388–2404. [Google Scholar] [CrossRef]
- Magee, L.; von Dadelszen, P. State-of-the art diagnosis and treatment of hypertension in pregnancy. Mayo Clin. Proc. 2018, 93, 1664–1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Firoz, T.; Magee, L.A.; MacDonell, K.; Payne, B.A.; Gordon, R.; Vidler, M.; von Dadelszen, P. Community Level Interventions for Pre-eclampsia (CLIP) Working Group. Oral antihypertensive therapy for severe hypertension in pregnancy and postpartum: A systematic review. BJOG 2014, 121, 1210–1218. [Google Scholar] [CrossRef] [Green Version]
- Buawangpong, N.; Teekachunhatean, S.; Koonrungsesomboon, N. Adverse pregnancy outcomes associated with first-trimester exposure to angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers: A systematic review and meta-analysis. Pharmacol. Res. Perspect. 2020, 8, e00644. [Google Scholar] [CrossRef]
- Fu, J.; Tomlinson, G.; Feig, D.S. Early Pregnancy Exposure to Angiotensin-Converting-Enzyme Inhibitors and Angiotensin Receptor Blockers Associated with Congenital Malformations and Stillbirths: A Systematic Review and Meta-analysis. Diabetes Metab. Res. Rev. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Majak, G.B.; Sandven, I.; Lorentzen, B.; Vangen, S.; Reisaeter, A.V.; Henriksen, T.; Michelsen, T.M. Pregnancy outcomes following maternal kidney transplantation: A national cohort study. Acta Obstet. Gynecol. Scand. 2016, 95, 1153–1161. [Google Scholar] [CrossRef]
- Josephson, M.A.; McKay, D.B. Pregnancy and kidney transplantation. Semin. Nephrol. 2011, 31, 100–110. [Google Scholar] [CrossRef]
- Phipps, E.; Prasanna, D.; Brima, W.; Jim, B. Preeclampsia updates in pathogenesis, definitions and gudelines. Clin. J. Am. Soc. Nephrol. 2016, 11, 1102–1113. [Google Scholar] [CrossRef] [Green Version]
- Schuster, J.; Cheng, S.B.; Padbury, J.; Sharma, S. Placental extracellular vesicles and pre-eclampsia. Am. J. Reprod. Immunol. 2021, 85, e13297. [Google Scholar] [CrossRef]
- Bandoli, G.; Palmsten, K.; Forbess Smith, C.J.; Chambers, C.D. A review of systemic corticosteroid use in pregnancy and the risk of select pregnancy and birth outcome. Rheum. Dis. Clin. N. Am. 2017, 43, 489–502. [Google Scholar] [CrossRef]
- Chakravarty, E.F.; Colón, I.; Langen, E.S.; Nix, D.A.; El-Sayed, Y.Y.; Genovese, M.C.; Druzin, M.L. Factors that predict prematurity and preeclampsia in pregnancies that are complicated by systemic lupus erythematosus. Am. J. Obstet. Gynecol. 2005, 192, 1897–1904. [Google Scholar] [CrossRef]
- Boyd, H.A.; Basit, S.; Harpsøe, M.C.; Wohlfahrt, J.; Jess, T. Inflammatory Bowel Disease and Risk of Adverse Pregnancy Outcomes. PLoS ONE 2015, 10, e0129567. [Google Scholar] [CrossRef]
- Palmsten, K.; Hernández-Diaz, S.; Kuriya, B.; Solomon, D.H.; Setoguchi, S. Use of Disease-Modifying Antirheumatic Drugs During Pregnancy and Risk of Preeclampsia. Arthritis Care Res. 2012, 64, 1730–1738. [Google Scholar] [CrossRef]
- Paziana, K.; Del Monaco, M.; Cardonick, E.; Moritz, M.; Keller, M.; Smith, B.; Coscia, L.; Armenti, V. Ciclosporin use during pregnancy. Drug Saf. 2013, 36, 279–294. [Google Scholar] [CrossRef]
- Le, H.L.; Francke, M.I.; Andrews, L.M.; de Winter, B.C.M.; van Gelder, T.; Hesselink, D.A. Usage of Tacrolimus and Mycophenolic Acid During Conception, Pregnancy, and Lactation, and Its Implications for Therapeutic Drug Monitoring: A Systematic Critical Review. Ther. Drug Monit. 2020, 42, 518–531. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Li, J.; Yan, J.; Dai, R.; Liu, L.; Chen, P.; Chen, X. Pregnancy outcomes in female patients exposed to cyclosporin-based versus tacrolimus-based immunosuppressive regimens after liver/kidney transplantation: A systematic review and meta-analysis. J. Clin. Pharm. Ther. 2021, 46, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Majak, G.B.; Reisæter, A.V.; Zucknick, M.; Lorentzen, B.; Vangen, S.; Henriksen, T.; Michelsen, T.M. Preeclampsia in kidney transplanted women; Outcomes and a simple prognostic risk score system. PLoS ONE 2017, 12, e0173420. [Google Scholar]
- Shah, P.B.; Samra, M.; Josephson, M.A. Preeclampsia risk in kidney donors and recipients. Curr. Hypertens. Rep. 2018, 20, 59. [Google Scholar] [CrossRef]
- Vannevel, V.; Claes, K.; Baud, D.; Vial, Y.; Golshayan, D.; Yoon, E.W.; Hodges, R.; Le Nepveu, A.; Kerr, P.G.; Kennedy, C.; et al. Preeclampsia and Long-term Renal Function in Women Who Underwent Kidney Transplantation. Obstet Gynecol. 2018, 131, 57–62. [Google Scholar] [CrossRef]
- Lucas, M.J.; Leveno, K.J.; Cunningham, F.G. A comparison of magnesium sulfate with phenytoin for the prevention of eclampsia. N. Engl. J. Med. 1995, 333, 201–205. [Google Scholar] [CrossRef]
- Saccone, G.; Berghella, V. Antenatal corticosteroids for maturity of term or nearterm fetuses: A systematic review and meta-analysis of randomized controlled trials. BMJ 2016, 355, i5044. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M.; Feinberg, B.B.; Burwick, R.M. Thrombotic microangiopathies of pregnancy: Differential diagnosis. Pregnancy Hypertens. 2018, 12, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Fakhouri, F.; Scully, M.; Provôt, F.; Blasco, M.; Coppo, P.; Noris, M.; Paizis, K.; Kavanagh, D.; Pène, F.; Quezada, S.; et al. Management of thrombotic microangiopathy in pregnancy and postpartum: Report from an international working group. Blood 2020, 136, 2103–2117. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.F.; Roman, J.; Wyble, A.; Pacheco, L.D. Pregnancy-Associated Atypical Hemolytic-Uremic Syndrome. AJP Rep. 2016, 6, e125–e128. [Google Scholar] [PubMed]
- Bruel, A.; Kavanagh, D.; Noris, M.; Delmas, Y.; Wong, E.K.S.; Bresin, E.; Provôt, F.; Brocklebank, V.; Mele, C.; Remuzzi, G.; et al. Hemolytic Uremic Syndrome in Pregnancy and Postpartum. Clin. J. Am. Soc. Nephrol. 2017, 12, 1237–1247. [Google Scholar] [CrossRef] [Green Version]
- Dines, V.; D’Costa, M.; Fidler, M.; Kattah, A. The role of kidney biopsy in diagnosis of preeclampsia in kidney transplant patients. Hypertens. Pregnancy 2020, 39, 418–422. [Google Scholar] [CrossRef]
- Kim, H.; Jeong, J.C.; Yang, J.; Yang, W.S.; Ahn, C.; Han, D.J.; Park, J.S.; Park, S.K. The optimal therapy of calcineurin inhibitors for pregnancy in kidney transplantation. Clin. Transplant. 2015, 29, 142–148. [Google Scholar] [CrossRef]
- Naesens, M.; Kuypers, D.R.; Sarwal, M. Calcineurin inhibitor nephrotoxicity. Clin. J. Am. Soc. Nephrol. 2009, 4, 481–508. [Google Scholar] [CrossRef] [Green Version]
- Epidis, K. Pregnancy in women with renal disease. Yes or no? Hippokratia 2011, 15 (Suppl. 1), 8–12. [Google Scholar]
- HAPO Study Cooperative Research Group; Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; et al. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar]
- Catalano, P.M.; McIntyre, H.D.; Cruickshank, J.K.; McCance, D.R.; Dyer, A.R.; Metzger, B.E.; Lowe, L.P.; Trimble, E.R.; Coustan, D.R.; Hadden, D.R.; et al. The hyperglycemia and adverse pregnancy outcome study: Associations of GDM and obesity with pregnancy outcomes. Diabetes Care 2012, 35, 780–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waters, T.P.; Dyer, A.R.; Scholtens, D.M.; Dooley, S.L.; Herer, E.; Lowe, L.P.; Oats, J.J.; Persson, B.; Sacks, D.A.; Metzger, B.E.; et al. Maternal and Neonatal Morbidity for Women Who Would Be Added to the Diagnosis of GDM Using IADPSG Criteria: A Secondary Analysis of the Hyperglycemia and Adverse Pregnancy Outcome Study. Diabetes Care 2016, 39, 2204–2210. [Google Scholar] [CrossRef] [Green Version]
- Coustan, D.R.; Lowe, L.P.; Metzger, B.E. The hyperglycemia and adverse pregnancy outcome (HAPO) study: Can we use the results as a basis for change? J. Matern. Fetal Neonatal Med. 2010, 23, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Lende, M.; Rijhsinghani, A. Gestational Diabetes: Overview with Emphasis on Medical Management. Int. J. Environ. Res. Public Health 2020, 17, 9573. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, Y.; Uslu, A. Pregnancy in patients with previous successful renal transplantation. Int. J. Gynaecol. Obstet. 2005, 90, 198–202. [Google Scholar] [CrossRef]
- Sturgiss, S.N.; Davison, J.M. Effect of pregnancy on the long-term function of renal allografts. An update. Am. J. Kidney Dis. 1995, 26, 54–56. [Google Scholar] [CrossRef]
- Fischer, T.; Neumayer, H.H.; Fischer, R.; Barenbrock, M.; Schobel, H.P.; Lattrell, B.C.; Jacobs, V.R.; Paepke, S.; Pildner von Steinburg, S.; Schmalfeldt, B.; et al. Effect of pregnancy on long-term kidney function in renal transplant recipients treated with cyclosporine and with azathioprine. Am. J. Transplant. 2005, 5, 2732–2739. [Google Scholar] [CrossRef]
- Rahamimov, R.; Ben-Haroush, A.; Wittenberg, C.; Mor, E.; Lustig, S.; Gafter, U.; Hod, M.; Bar, J. Pregnancy in renal transplant recipients: Long-term effect on patient and graft survival. A single-center experience. Transplantation 2006, 81, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, M.E.; El-Baghdadi, L.A.; Badawy, A.M.; Bakr, M.A.; Sobhe, M.A.; Ghoneim, M.A. Pregnancy outcome after renal allograft transplantation: 15 years’ experience. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 121, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Levidiotis, V.; Chang, S.; McDonald, S. Pregnancy and maternal outcomes among kidney transplant recipients. J. Am. Soc. Nephrol. 2009, 20, 2433–2440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Buren, M.C.; Schellekens, A.; Groenhof, T.K.J.; van Reekum, F.; van de Wetering, J.; Paauw, N.D.; Lely, A.T. Long-term Graft Survival and Graft Function Following Pregnancy in Kidney Transplant Recipients: A Systematic Review and Meta-analysis. Transplantation 2020, 104, 1675–1685. [Google Scholar] [CrossRef]
- Thompson, B.C.; Kingdon, E.J.; Tuck, S.M.; Fernando, O.N.; Sweny, P. Pregnancy in renal transplant recipients: The Royal Free Hospital experience. QJM 2003, 96, 837–844. [Google Scholar] [CrossRef] [Green Version]
- You, J.Y.; Kim, M.K.; Choi, S.J.; Oh, S.Y.; Kim, S.J.; Kim, J.H.; Oh, H.Y.; Roh, C.R. Predictive factors for adverse pregnancy outcomes after renal transplantation. Clin. Transplant. 2014, 28, 699–706. [Google Scholar] [CrossRef]
- Covella, B.; Vinturache, A.E.; Cabiddu, G.; Attini, R.; Gesualdo, L.; Versino, E.; Piccoli, G.B. A systematic review and meta-analysis indicate long-term risk of chronic and end-stage kidney disease after preeclampsia. Kidney Int. 2019, 96, 711–727. [Google Scholar] [CrossRef]
- Solano, M.E.; Arck, P.C. Steroids, pregnancy and fetal development. Front. Immunol. 2020, 10, 3017. [Google Scholar] [CrossRef]
- Levitz, M.; Jansen, V.; Dancis, J. The transfer and metabolism of corticosteroids in the perfused human placenta. Am. J. Obstet. Gynecol. 1978, 132, 363–366. [Google Scholar] [CrossRef]
- Brown, R.W.; Chapman, K.E.; Edwards, C.R.; Seckl, J.R. Human placental 11 beta-hydroxysteroid dehydrogenase: Evidence for and partial purification of a distinct NAD-dependent isoform. Endocrinology 1993, 132, 2614–2621. [Google Scholar] [CrossRef]
- Busada, J.T.; Cidlowski, J.A. Mechanisms of glucocorticoid action during development. Curr. Top. Dev. Biol. 2017, 125, 147–170. [Google Scholar]
- Goedhart, G.; Vrijkotte, T.G.; Roseboom, T.J.; van der Wal, M.F.; Cuijpers, P.; Bonsel, G.J. Maternal cortisol and offspring birthweight: Results from a large prospective cohort study. Psychoneuroendocrinology 2010, 35, 644–652. [Google Scholar] [CrossRef]
- Bloom, S.L.; Sheffield, J.S.; McIntire DDLeveno, K.J. Antenatal dexamethasone and decreased birth weight. Obstet. Gynecol. 2001, 97, 485–490. [Google Scholar]
- Reynolds, R.M. Programming effects of glucocorticoids. Clin. Obstet. Gynecol. 2013, 56, 602–609. [Google Scholar] [CrossRef]
- Moisiadis, V.G.; Matthews, S.G. Glucocorticoids and fetal programming part 1: Outcomes. Nat. Rev. Endocrinol. 2014, 10, 391–402. [Google Scholar] [CrossRef]
- McCalla, C.; Nacharaju, V.L.; Muneyyirci-Delale, O.; Glasgow, S.; Feldman, J.G. Placental 11β-hydroxysteroid dehydrogenase activity in normotensive and pre-eclamptic pregnancies. Steroids 1998, 63, 511–515. [Google Scholar] [CrossRef]
- Chambers, C.D.; Tutuncu, Z.N.; Johnson, D.; Jones, K.L. Human pregnancy safety for agents used to treat rheumatoid arthritis: Adequacy of available information and strategies for developing post-marketing data. Arthritis Res. Ther. 2006, 8, 215. [Google Scholar] [CrossRef]
- Bay Bjørn, A.M.; Ehrenstein, V.; Hundborg, H.H.; Nohr, E.A.; Sørensen, H.T.; Nørgaard, M. Use of corticosteroids in early pregnancy is not associated with risk of oral clefts and other congenital malformations in offspring. Am. J. Ther. 2014, 21, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Armenti, V.T. Pregnancy after transplantation. Milestones and assessment of risk. Am. J. Transpl. 2011, 11, 2275–2276. [Google Scholar] [CrossRef] [PubMed]
- Venkataramanan, R.; Koneru, B.; Wang, C.C.; Burckart, G.J.; Caritis, S.N.; Starzl, T.E. Cyclosporine and its metabolites in mother and baby. Transplantation 1988, 46, 468–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pávek, P.; Fendrich, Z.; Staud, F.; Malákova, J.; Brozmanová, H.; Láznícek, M.; Semecký, V.; Grundmann, M.; Palicka, V. Influence of P-glycoprotein on the transplacental passage of cyclosporine. J. Pharm. Sci. 2001, 90, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Janssen, N.M.; Genta, M.S. The effects of immunosuppressive and anti-inflammatory medications on fertility, pregnancy and lactation. Arch. Intern. Med. 2000, 160, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Gotestam Skorpen, C.; Hoeltzenbein, M.; Tincani, A.; Fischer-Betz, R.; Elefante, E.; Chambers, C.; da Silva, J.; Nelson-Piercy, C.; Cetin, I.; Costedoat-Chalumeau, N.; et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann. Rheum. Dis. 2016, 75, 795–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hebert, M.F.; Zheng, S.; Hays, K.; Shen, D.D.; Davis, C.L.; Umans, J.G.; Miodovnik, M.; Thummel, K.E.; Easterling, T.R. Interpreting tacrolimus concentrations during pregnancy and postpartum. Transplantation 2013, 95, 908–915. [Google Scholar] [CrossRef] [Green Version]
- Sibanda, N.; Briggs, J.D.; Davison, J.M.; Johnson, R.J.; Rudge, C.J. Pregnancy after organ transplantation: A report from the UK Transplant Pregnancy Registry. Transplantation 2007, 83, 1301–1307. [Google Scholar] [CrossRef]
- Piccoli, G.B.; Cabiddu, G.; Attini, R.; Gerbino, M.; Todeschini, P.; Perrino, M.L.; Manzione, A.M.; Piredda, G.B.; Gnappi, E.; Caputo, F.; et al. Pregnancy outcomes after kidney graft in Italy: Are the changes over time the result of different therapies or of different policies? A nationwide survey (1978–2013). Nephrol. Dial. Transpl. 2016, 31, 1957–1965. [Google Scholar] [CrossRef]
- Hutson, J.R.; Lubetsky, A.; Walfisch, A.; Ballios, B.G.; Garcia-Bournissen, F.; Koren, G. The transfer of 6-mercaptopurine in the dually perfused human placenta. Reprod. Toxicol. 2011, 32, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Belizna, C.; Meroni, P.L.; Shoenfeld, Y.; Devreese, K.; Alijotas-Reig, J.; Esteve-Valverde, E.; Chighizola, C.; Pregnolato, F.; Cohen, H.; Fassot, C.; et al. In utero exposure to Azathioprine in autoimmune disease. Where do we stand? Autoimmun. Rev. 2020, 19, 102925. [Google Scholar] [CrossRef]
- Ponticelli, C. The pros and cons of mTOR inhibitors in kidney transplantation. Expert Rev. Clin. Immunol. 2014, 10, 295–305. [Google Scholar] [CrossRef]
- Chu, S.H.; Liu, K.L.; Chiang, Y.J.; Wang, H.H.; Lai, P.C. Sirolimus used during pregnancy in a living related renal transplant recipient: A case report. Transpl. Proc. 2008, 40, 2446–2448. [Google Scholar] [CrossRef]
- Framarino dei Malatesta, M.; Corona, L.E.; De Luca, L.; Rocca, B.; Maria Manzia, T.; Orlando, G.; Tisone, G.; Iaria, G. Successful pregnancy in a living-related kidney transplant recipient who received sirolimus throughout the whole gestation. Transplantation 2011, 91, e69–e71. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, M.; Kojima, T.; Koyama, M.; Sazawa, A.; Yamada, T.; Minakami, H. Everolimus in pregnancy: Case report and literature review. J. Obstet. Gynaecol. Res. 2017, 43, 1350–1352. [Google Scholar] [CrossRef] [PubMed]
- Veroux, M.; Corona, D.; Veroux, P. Pregnancy under everolimus-based immunosuppression. Transpl. Int. 2011, 24, e115–e117. [Google Scholar] [CrossRef] [PubMed]
- Margoles, H.R.; Gomez-Lobo, V.; Veis, J.H.; Sherman, M.J.; Moore, J., Jr. Successful maternal and fetal outcome in a kidney transplant patient with everolimus exposure throughout pregnancy: A case report. Transpl. Proc. 2014, 46, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Athanassakis, I.; Chaouat, G.; Wegmann, T.G. The effects of anti-CD4 and anti-CD8 antibody treatment on placental growth and function in allogeneic and syngeneic murine pregnancy. Cell. Immunol. 1990, 129, 13–21. [Google Scholar] [CrossRef]
- Kutzler, H.L.; Ye, X.; Rochon, C.; Martin, S.T. Administration of Antithymocyte Globulin (Rabbit) to Treat a Severe, Mixed Rejection Episode in a Pregnant Renal Transplant Recipient. Pharmacotherapy 2016, 36, e18–e22. [Google Scholar] [CrossRef]
- Combs, J.; Kagan, A.; Boelkins, M.; Coscia, L.; Moritz, M.; Hoffmann, R.M. Belatacept during pregnancy in renal transplant recipients: Two case reports. Am. J. Transpl. 2018, 18, 2079–2082. [Google Scholar] [CrossRef] [Green Version]
- Klintmalm, G.B.; Gunby, R.T., Jr. Successful Pregnancy in a Liver Transplant Recipient on Belatacept. Liver Transpl. 2020, 26, 1193–1194. [Google Scholar] [CrossRef]
- Chakravarty, E.F.; Murray, E.R.; Kelman, A.; Farmer, P. Pregnancy outcomes after maternal exposure to rituximab. Blood 2011, 117, 1499–1506. [Google Scholar] [CrossRef] [Green Version]
- Das, G.; Damotte, V.; Gelfand, J.M.; Bevan, C.; Cree, B.A.C.; Do, L.; Green, A.J.; Hauser, S.L.; Bove, R. Rituximab before and during pregnancy: A systematic review, and a case series in MS and NMOSD. Neurol. Neuroimmunol. Neuroinflamm. 2018, 19, e453. [Google Scholar] [CrossRef] [Green Version]
- Ostensen, M. Safety issues of biologics in pregnant patients with rheumatic diseases. Ann. N. Y. Acad. Sci. 2014, 1317, 32–38. [Google Scholar] [CrossRef]
- Levy, R.A.; de Jesús, G.R.; de Jesús, N.R.; Klumb, E.M. Critical review of the current recommendations for the treatment of systemic inflammatory rheumatic diseases during pregnancy and lactation. Autoimmun. Rev. 2016, 15, 955–963. [Google Scholar] [CrossRef]
- Hallstensen, R.F.; Bergseth, G.; Foss, S.; Jæger, S.; Gedde-Dahl, T.; Holt, J.; Christiansen, D.; Lau, C.; Brekke, O.L.; Armstrong, E.; et al. Eculizumab treatment during pregnancy does not affect the complement system activity of the newborn. Immunobiology 2015, 220, 452–459. [Google Scholar] [CrossRef]
- Kelly, R.J.; Höchsmann, B.; Szer, J.; Kulasekararaj, A.; de Guibert, S.; Röth, A.; Weitz, I.C.; Armstrong, E.; Risitano, A.M.; Patriquin, C.J.; et al. Eculizumab in Pregnant Patients with Paroxysmal Nocturnal Hemoglobinuria. N. Engl. J. Med. 2015, 373, 1032–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarno, L.; Tufano, A.; Maruotti, G.M. Eculizumab in pregnancy: A narrative review. J. Nephrol. 2019, 32, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Hoeltzenbein, M.; Elefant, E.; Vial, T.; Finkel-Pekarsky, V.; Stephens, S.; Clementi, M.; Allignol, A.; Weber-Schoendorfer, C.; Schaefer, C. Teratogenicity of mycophenolate confirmed in a prospective study of the European Network of Teratology Information Services. Am. J. Med. Genet. A 2012, 58, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.C.; Cristiano, E.; Villanueva, M.; Bonora, M.L.; Berguio, N.; Tocci, A.; Groisman, B.; Bidondo, M.P.; Liascovich, R.; Barbero, P. Esophageal atresia and prenatal exposure to mycophenolate. Reprod. Toxicol. 2014, 50, 117–121. [Google Scholar] [CrossRef]
- Coscia, L.A.; Armenti, D.; King, R.W.; Sifontis, N.M.; Constantinescu, S.; Moritz, M.J. Update on the Teratogenicity of Maternal Mycophenolate Mofetil. J. Pediatr. Genet. 2015, 4, 42–55. [Google Scholar]
- Klieger-Grossmann, C.; Chitayat, D.; Lavign, S.; Kao, K.; Garcia-Bournissen, F.; Quinn, D.; Luo, V.; Sermer, M.; Riordan, S.; Laskin, C.; et al. Prenatal exposure to mycophenolate mofetil: An updated estimate. J. Obstet. Gynaecol. Can. 2010, 32, 794–797. [Google Scholar] [CrossRef]
- Kylat, R.I. What is the teratogenic risk of mycophenolate? J. Pediatr. Genet. 2017, 6, 111–114. [Google Scholar] [CrossRef]
- King, R.W.; Baca, M.J.; Armenti, V.T.; Kaplan, B. Pregnancy Outcomes Related to Mycophenolate Exposure in Female Kidney Transplant Recipients. Am. J. Transpl. 2017, 17, 151–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colla, L.; Diena, D.; Rossetti, M.; Manzione, A.M.; Marozio, L.; Benedetto, C.; Biancone, L. Immunosuppression in pregnant women with renal disease: Review of the latest evidence in the biologic’s era. J. Nephrol. 2018, 31, 361–383. [Google Scholar] [CrossRef] [PubMed]
- Kirshon, B.; Wasserstrum, N.; Willis, R.; Herman, G.E.; McCabe, E.R. Teratogenic effects of first-trimester cyclophosphamide therapy. Obstet. Gynecol. 1988, 72, 462–464. [Google Scholar] [PubMed]
- Grijalva-Flores, J.; Guerrero-Romero, F. Klippel-Feil syndrome in a boy exposed inadvertently to cyclophosphamide during pregnancy: A case report. Birth Defects Res. A Clin. Mol. Teratol. 2012, 94, 249–252. [Google Scholar]
- Rengasamy, P. Congenital Malformations Attributed to Prenatal Exposure to Cyclophosphamide. Anticancer Agents Med. Chem. 2017, 17, 1211–1227. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.C.; Kumar, V. Pregnancy in a kidney transplant patient. Clin. J. Am. Soc. Nephrol. 2020, 15, 120–122. [Google Scholar] [CrossRef]
- Gordon, C.E.; Tatsis, V. Shearing-force injury of a kidney transplant graft during cesarean section: A case report and review of the literature. BMC Nephrol. 2019, 20, 1852. [Google Scholar] [CrossRef] [Green Version]
| Complication | Incidence | Consequences |
|---|---|---|
| Infection | Very common. Urinary tract infection (often asymptomatic) may affect most transplant women. | Urinary tract infections can increase the risk of preeclampsia. Pyelonephritis is an uncommon but severe complication. The risk of viral infection is elevated in case of early pregnancy. Infection may lead to graft dysfunction. |
| Hypertension | Many transplanted women are already hypertensive before pregnancy. | Hypertension can predispose to preeclampsia and is a main risk factor for cardiovascular disease, cerebrovascular disease, and graft dysfunction. |
| Preeclampsia | The incidence is higher in patients with poor graft function or hypertension. | Preeclampsia may result in damage to the kidneys, liver, lung, heart, or eyes, and may cause a stroke or other brain injury. |
| Gestational diabetes mellitus | About 16% | A maternal and neonate risk factor of adverse events during pregnancy. |
| Acute rejection | About 10% | Risk factor for graft dysfunction. Infection may develop after aggressive immunosuppressive treatment. |
| Glucocorticoids (GC) | GC can cross the placenta but are metabolized by 11 β-HSD2 to inactive products with the exception of dexamethasone and betamethasone. Fetal toxicity cannot be ruled out (Class C according to FDA). | Minimal risk of oral-facial cleft. It is unlikely that moderate doses of GC lead to fetal hypothalamic-pituitary-adrenal axis dysfunction or interfere with the early life programming, since 11β-hydroxysteroid dehydrogenase 2 is a major barrier against cortisol transfer to the fetus. Concern remains about the fetal toxicity when the mothers receive prolonged treatments with high-dose GC. |
| Calcineurin inhibitors (CNI) | CNI metabolites can be seen in the placenta. The CNI maternal–fetal transplacental passage is influenced by the activity of P-glycoprotein that pumps CNI out of the trophoblast cells of placenta and restricts its passage across the placental barrier. CNI are listed in Class C. | The relatively high number of premature births that has been reported may be partially explained by an obstetric policy favoring earlier delivery. Nonetheless, long-term effects in humans prenatally exposed to CNIs require further evaluation. |
| Azathioprine (AZA) | Placenta is a barrier to 6-mercaptopurine the main metabolite of AZA. This explains the lack of teratogenicity of AZA. The FDA classified AZA as a drug at potential risk of teratogenic effects (Class C) based on animal studies. | The risk of congenital anomalies in offspring are similar to those found in general population, but there is a higher incidence of prematurity and lower weight at birth in AZA-treated pregnant women. There is also an increased risk of materno-fetal infections, especially CMV infection. The use of AZA is considered safe. |
| Mycophenolic acid (MPA) | MPA during pregnancy is associated with an increased risk of congenital malformations. MPA is considered as class D (Evidence of risk to human fetus) by FDA. | Women being considered for treatment with MPA should always have a negative pregnancy test. There is an increased risk of pregnancy loss and congenital malformations with the use of MPA during pregnancy. If pregnancy occurs MPA should be stopped. The later MPA is discontinued the higher is the risk of complications. |
| mTOR inhibitors (mTORi) | Animal reproduction studies have shown an adverse effect on the fetus. There are not adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks; mTORi are considered as class C by FDA. | The mTORi exposure during pregnancy does not appear to be associated with an increased risk or a pattern of birth defects. |
| Rituximab (RTX) | Data on RTX during pregnancy are scarce. RTX contains an IgG and can cross the placenta. RTX is considered in class C. | The administration of RTX to a pregnant woman is discouraged unless the benefits outweigh the potential risk for the fetus. |
| Eculizumab | There is a selective transport of unbound eculizumab across the placenta. However, the levels observed in umbilical cord blood samples do not affect the concentrations of complement in newborns. | The use of eculizumab to pregnant women with paroxysmal hemoglobinuria nocturnal is associated with a high rate of fetal survival and a low rate of maternal complications. Therefore, eculizumab may be regarded as safe in pregnancy. |
| Belatacept | There are not formal studies for the use of Belimumab in pregnant women. | Anecdotal cases of successful pregnancy with the use of belatacept in transplant women have been reported. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponticelli, C.; Zaina, B.; Moroni, G. Planned Pregnancy in Kidney Transplantation. A Calculated Risk. J. Pers. Med. 2021, 11, 956. https://doi.org/10.3390/jpm11100956
Ponticelli C, Zaina B, Moroni G. Planned Pregnancy in Kidney Transplantation. A Calculated Risk. Journal of Personalized Medicine. 2021; 11(10):956. https://doi.org/10.3390/jpm11100956
Chicago/Turabian StylePonticelli, Claudio, Barbara Zaina, and Gabriella Moroni. 2021. "Planned Pregnancy in Kidney Transplantation. A Calculated Risk" Journal of Personalized Medicine 11, no. 10: 956. https://doi.org/10.3390/jpm11100956
APA StylePonticelli, C., Zaina, B., & Moroni, G. (2021). Planned Pregnancy in Kidney Transplantation. A Calculated Risk. Journal of Personalized Medicine, 11(10), 956. https://doi.org/10.3390/jpm11100956