U-Shaped Association between Waist-to-Hip Ratio and All-Cause Mortality in Stage 3–5 Chronic Kidney Disease Patients with Body Mass Index Paradox
Abstract
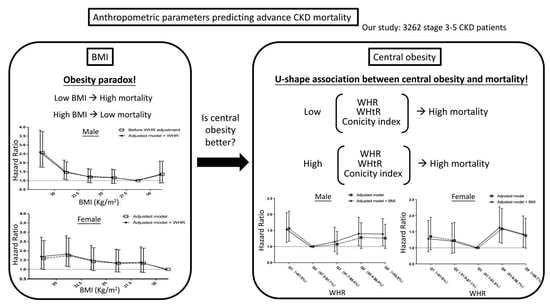
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Collection of Demographics, Medical and Laboratory Data
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Patients by WHR Quintiles
3.2. Odds Ratios for Malnutrition–Inflammation Complex According to WHR
3.3. Association between BMI and All-Cause Mortality
3.4. Association between WHR and Mortality
3.5. Association between Waist-to-Height Ratio and Mortality
3.6. Association between Conicity Index and Mortality
3.7. Association between BMI and WHR
3.8. The Association of Metabolic Syndrome Components and Inflammation with Mortality
3.9. The Association between Malnutrition-Inflammation Score and Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fox, C.S.; Larson, M.G.; Leip, E.P.; Culleton, B.; Wilson, P.W.; Levy, D. Predictors of new-onset kidney disease in a community-based population. JAMA 2004, 291, 844–850. [Google Scholar] [CrossRef] [Green Version]
- Berrington de Gonzalez, A.; Hartge, P.; Cerhan, J.R.; Flint, A.J.; Hannan, L.; MacInnis, R.J.; Moore, S.C.; Tobias, G.S.; Anton-Culver, H.; Freeman, L.B.; et al. Body-mass index and mortality among 1.46 million white adults. N. Engl. J. Med. 2010, 363, 2211–2219. [Google Scholar] [CrossRef] [Green Version]
- Gu, D.; He, J.; Duan, X.; Reynolds, K.; Wu, X.; Chen, J.; Huang, G.; Chen, C.S.; Whelton, P.K. Body weight and mortality among men and women in China. JAMA 2006, 295, 776–783. [Google Scholar] [CrossRef] [Green Version]
- Whitlock, G.; Lewington, S.; Sherliker, P.; Clarke, R.; Emberson, J.; Halsey, J.; Qizilbash, N.; Collins, R.; Peto, R. Body-mass index and cause-specific mortality in 900 000 adults: Collaborative analyses of 57 prospective studies. Lancet 2009, 373, 1083–1096. [Google Scholar] [CrossRef] [Green Version]
- Kalantar-Zadeh, K.; Abbott, K.C.; Salahudeen, A.K.; Kilpatrick, R.D.; Horwich, T.B. Survival advantages of obesity in dialysis patients. Am. J. Clin. Nutr. 2005, 81, 543–554. [Google Scholar] [CrossRef]
- Huang, J.C.; Lin, H.Y.; Lim, L.M.; Chen, S.C.; Chang, J.M.; Hwang, S.J.; Tsai, J.C.; Hung, C.C.; Chen, H.C. Body mass index, mortality, and gender difference in advanced chronic kidney disease. PLoS ONE 2015, 10, e0126668. [Google Scholar] [CrossRef]
- Van Gaal, L.F.; Mertens, I.L.; De Block, C.E. Mechanisms linking obesity with cardiovascular disease. Nature 2006, 444, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Wildman, R.P.; Muntner, P.; Reynolds, K.; McGinn, A.P.; Rajpathak, S.; Wylie-Rosett, J.; Sowers, M.R. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: Prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch. Intern. Med. 2008, 168, 1617–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karelis, A.D.; St-Pierre, D.H.; Conus, F.; Rabasa-Lhoret, R.; Poehlman, E.T. Metabolic and body composition factors in subgroups of obesity: What do we know? J. Clin. Endocrinol. Metab. 2004, 89, 2569–2575. [Google Scholar] [CrossRef]
- Després, J.P.; Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Pischon, T.; Boeing, H.; Hoffmann, K.; Bergmann, M.; Schulze, M.B.; Overvad, K.; van der Schouw, Y.T.; Spencer, E.; Moons, K.G.; Tjønneland, A.; et al. General and abdominal adiposity and risk of death in Europe. N. Engl. J. Med. 2008, 359, 2105–2120. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Bautista, L.; Franzosi, M.G.; Commerford, P.; Lang, C.C.; Rumboldt, Z.; Onen, C.L.; Lisheng, L.; et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: A case-control study. Lancet 2005, 366, 1640–1649. [Google Scholar] [CrossRef]
- Streng, K.W.; Voors, A.A.; Hillege, H.L.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; Filippatos, G.; Metra, M.; Ng, L.L.; Ponikowski, P.; et al. Waist-to-hip ratio and mortality in heart failure. Eur. J. Heart Fail. 2018, 20, 1269–1277. [Google Scholar] [CrossRef]
- Shah, R.; Gayat, E.; Januzzi, J.L., Jr.; Sato, N.; Cohen-Solal, A.; diSomma, S.; Fairman, E.; Harjola, V.P.; Ishihara, S.; Lassus, J.; et al. Body mass index and mortality in acutely decompensated heart failure across the world: A global obesity paradox. J. Am. Coll. Cardiol. 2014, 63, 778–785. [Google Scholar] [CrossRef] [Green Version]
- Postorino, M.; Marino, C.; Tripepi, G.; Zoccali, C. Abdominal obesity and all-cause and cardiovascular mortality in end-stage renal disease. J. Am. Coll. Cardiol. 2009, 53, 1265–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.S.; Han, K.D.; Choi, H.S.; Bae, E.H.; Ma, S.K.; Kim, S.W. Association of Body Mass Index and Waist Circumference with All-Cause Mortality in Hemodialysis Patients. J. Clin. Med. 2020, 9, 1289. [Google Scholar] [CrossRef]
- Fitzpatrick, J.; Sozio, S.M.; Jaar, B.G.; McAdams-DeMarco, M.A.; Estrella, M.M.; Tereshchenko, L.G.; Monroy-Trujillo, J.M.; Parekh, R.S. Association of Abdominal Adiposity with Cardiovascular Mortality in Incident Hemodialysis. Am. J. Nephrol. 2018, 48, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Stolic, R.; Trajkovic, G.; Jovanovic, A.; Peric, V.; Stolic, D.; Sovtic, S.; Subaric-Gorgieva, G. Association of metabolic changes with mortality of patients treated by peritoneal dialysis or hemodialysis. Ren. Fail. 2010, 32, 778–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsayed, E.F.; Tighiouart, H.; Weiner, D.E.; Griffith, J.; Salem, D.; Levey, A.S.; Sarnak, M.J. Waist-to-hip ratio and body mass index as risk factors for cardiovascular events in CKD. Am. J. Kidney Dis. 2008, 52, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Kramer, H.; Shoham, D.; McClure, L.A.; Durazo-Arvizu, R.; Howard, G.; Judd, S.; Muntner, P.; Safford, M.; Warnock, D.G.; McClellan, W. Association of waist circumference and body mass index with all-cause mortality in CKD: The REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study. Am. J. Kidney Dis. 2011, 58, 177–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, E.; Campbell, K.; Gobe, G.; Hawley, C.; Isbel, N.; Johnson, D.W. Association of anthropometric measures with kidney disease progression and mortality: A retrospective cohort study of pre-dialysis chronic kidney disease patients referred to a specialist renal service. BMC Nephrol. 2016, 17, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rambod, M.; Bross, R.; Zitterkoph, J.; Benner, D.; Pithia, J.; Colman, S.; Kovesdy, C.P.; Kopple, J.D.; Kalantar-Zadeh, K. Association of Malnutrition-Inflammation Score with quality of life and mortality in hemodialysis patients: A 5-year prospective cohort study. Am. J. Kidney Dis. 2009, 53, 298–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Hendryx, M.; Laddu, D.; Phillips, L.S.; Chlebowski, R.; LeBlanc, E.S.; Allison, D.B.; Nelson, D.A.; Li, Y.; Rosal, M.C.; et al. Racial and Ethnic Differences in Anthropometric Measures as Risk Factors for Diabetes. Diabetes Care 2019, 42, 126–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdem, M.; Möckel, D.; Jumpertz, S.; John, C.; Fragoulis, A.; Rudolph, I.; Wulfmeier, J.; Springer, J.; Horn, H.; Koch, M.; et al. Macrophages protect against loss of adipose tissue during cancer cachexia. J. Cachexia Sarcopenia Muscle 2019, 10, 1128–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charette, N.; Vandeputte, C.; Ameye, L.; Bogaert, C.V.; Krygier, J.; Guiot, T.; Deleporte, A.; Delaunoit, T.; Geboes, K.; Van Laethem, J.L.; et al. Prognostic value of adipose tissue and muscle mass in advanced colorectal cancer: A post hoc analysis of two non-randomized phase II trials. BMC Cancer 2019, 19, 134. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; McLerran, D.F.; Rolland, B.; Zhang, X.; Inoue, M.; Matsuo, K.; He, J.; Gupta, P.C.; Ramadas, K.; Tsugane, S.; et al. Association between body-mass index and risk of death in more than 1 million Asians. N. Engl. J. Med. 2011, 364, 719–729. [Google Scholar] [CrossRef] [Green Version]
- Consultation, W. Waist Circumference and Waist-Hip Ratio; Report of a WHO Expert Consultation; World Health Organization: Geneva, Switzerland, 2008; Volume 2008, pp. 8–11. [Google Scholar]
- Kalantar-Zadeh, K.; Kopple, J.D.; Block, G.; Humphreys, M.H. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am. J. Kidney Dis. 2001, 38, 1251–1263. [Google Scholar] [CrossRef] [Green Version]
- Amparo, F.C.; Kamimura, M.A.; Molnar, M.Z.; Cuppari, L.; Lindholm, B.; Amodeo, C.; Carrero, J.J.; Cordeiro, A.C. Diagnostic validation and prognostic significance of the Malnutrition-Inflammation Score in nondialyzed chronic kidney disease patients. Nephrol. Dial. Transplant. 2015, 30, 821–828. [Google Scholar] [CrossRef] [Green Version]
- Chiang, H.P.; Chiu, Y.W.; Lee, J.J.; Hung, C.C.; Hwang, S.J.; Chen, H.C. Blood pressure modifies outcomes in patients with stage 3 to 5 chronic kidney disease. Kidney Int. 2020, 97, 402–413. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Copeland, W.K.; Vedanthan, R.; Grant, E.; Lee, J.E.; Gu, D.; Gupta, P.C.; Ramadas, K.; Inoue, M.; Tsugane, S.; et al. Association between body mass index and cardiovascular disease mortality in east Asians and south Asians: Pooled analysis of prospective data from the Asia Cohort Consortium. BMJ 2013, 347, f5446. [Google Scholar] [CrossRef] [Green Version]
- Kovesdy, C.P.; Anderson, J.E.; Kalantar-Zadeh, K. Paradoxical association between body mass index and mortality in men with CKD not yet on dialysis. Am. J. Kidney Dis. 2007, 49, 581–591. [Google Scholar] [CrossRef]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Wang, W.L.; Liang, S.; Zhu, F.L.; Liu, J.Q.; Chen, X.M.; Cai, G.Y. Association of the malnutrition-inflammation score with anthropometry and body composition measurements in patients with chronic kidney disease. Ann. Palliat. Med. 2019, 8, 596–603. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Heimbürger, O.; Paultre, F.; Diczfalusy, U.; Wang, T.; Berglund, L.; Jogestrand, T. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999, 55, 1899–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antón-Pérez, G.; Santana-Del-Pino, Á.; Henríquez-Palop, F.; Monzón, T.; Sánchez, A.Y.; Valga, F.; Morales-Umpierrez, A.; García-Cantón, C.; Rodríguez-Pérez, J.C.; Carrero, J.J. Diagnostic Usefulness of the Protein Energy Wasting Score in Prevalent Hemodialysis Patients. J. Ren. Nutr. 2018, 28, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A.; Cano, N.J.; Franch, H.; Fouque, D.; Himmelfarb, J.; Kalantar-Zadeh, K.; Kuhlmann, M.K.; Stenvinkel, P.; TerWee, P.; Teta, D.; et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: A consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013, 84, 1096–1107. [Google Scholar] [CrossRef] [Green Version]
- Mechanick, J.I.; Zhao, S.; Garvey, W.T. The Adipokine-Cardiovascular-Lifestyle Network: Translation to Clinical Practice. J. Am. Coll. Cardiol. 2016, 68, 1785–1803. [Google Scholar] [CrossRef] [PubMed]
- Caulin-Glaser, T.; Farrell, W.J.; Pfau, S.E.; Zaret, B.; Bunger, K.; Setaro, J.F.; Brennan, J.J.; Bender, J.R.; Cleman, M.W.; Cabin, H.S.; et al. Modulation of circulating cellular adhesion molecules in postmenopausal women with coronary artery disease. J. Am. Coll. Cardiol. 1998, 31, 1555–1560. [Google Scholar] [CrossRef] [Green Version]
- Vogt, B.P.; Caramori, J.C.T. Recognition of visceral obesity beyond body fat: Assessment of cardiovascular risk in chronic kidney disease using anthropometry. Nutrire 2017, 42, 19. [Google Scholar] [CrossRef]
- Ashwell, M.; Gunn, P.; Gibson, S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: Systematic review and meta-analysis. Obes. Rev. 2012, 13, 275–286. [Google Scholar] [CrossRef]
- Welborn, T.A.; Dhaliwal, S.S. Preferred clinical measures of central obesity for predicting mortality. Eur. J. Clin. Nutr. 2007, 61, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.I.; Gao, Y.; Anugu, P.; Anugu, A.; Correa, A. Obesity and overall mortality: Findings from the Jackson Heart Study. BMC Public Health 2021, 21, 50. [Google Scholar] [CrossRef] [PubMed]
- Rost, S.; Freuer, D.; Peters, A.; Thorand, B.; Holle, R.; Linseisen, J.; Meisinger, C. New indexes of body fat distribution and sex-specific risk of total and cause-specific mortality: A prospective cohort study. BMC Public Health 2018, 18, 427. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Waist-to-Hip Ratio | |||||||
|---|---|---|---|---|---|---|---|
| Variable | All | Q1 | Q2 | Q3 | Q4 | Q5 | p Value (ANOVA) |
| WHR (male) (%) | <87.5 | 87.5–91.7 | 91.7–95.4 | 95.4–99.9 | >99.9 | ||
| WHR (female) (%) | <81.0 | 81.0–87.1 | 87.1–91.8 | 91.8–98.7 | >98.7 | ||
| No. of patients | 3262 | 651 (20.0%) | 653 (20.0%) | 653 (20.0%) | 653 (20.0%) | 652 (20.0%) | |
| Demographics and medical history | |||||||
| Age (years) | 63.5 (13.4) | 58.4 (15.2) | 62.1 (12.8) | 63.5 (12.6) | 66.1 (12.2) | 67.5 (12.3) | <0.001 |
| Sex (female) | 1369 (42.0%) | 273 (41.9%) | 274 (42.0%) | 274 (42.0%) | 274 (42.0%) | 274 (42.0%) | 1.000 |
| Comorbidity | |||||||
| Cardiovascular disease | 847 (26.0%) | 135 (20.7%) | 145 (22.2%) | 162 (24.8%) | 186 (28.5%) | 219 (33.5%) | <0.001 |
| Ischemic heart disease | 485 (14.9%) | 73 (11.2%) | 95 (14.5%) | 92 (14.1%) | 111 (17.0%) | 114 (17.5%) | 0.011 |
| Congestive heart disease | 407 (12.5%) | 63 (9.7%) | 63 (9.6%) | 72 (11.0%) | 92 (14.1%) | 117 (17.9%) | <0.001 |
| Cerebrovascular disease | 554 (17.0%) | 89 (13.7%) | 93 (14.2%) | 109 (16.7%) | 125 (19.1%) | 138 (21.1%) | <0.001 |
| Hypertension | 2157 (66.1%) | 410 (63.0%) | 432 (66.2%) | 444 (68.0%) | 429 (65.7%) | 442 (67.7%) | 0.324 |
| Diabetes mellitus | 1641 (50.35%) | 278 (42.7%) | 276 (42.3%) | 320 (49.0%) | 356 (54.5%) | 411 (62.9%) | <0.001 |
| Hyperuricemia | 558 (17.1%) | 99 (15.2%) | 94 (14.4%) | 127 (19.4%) | 122 (18.7%) | 116 (17.8%) | 0.063 |
| Severe liver disease | 160 (4.9%) | 34 (5.2%) | 31 (4.7%) | 28 (4.3%) | 37 (5.7%) | 30 (4.6%) | 0.799 |
| Cancer | 289 (8.9%) | 53 (8.1%) | 68 (10.4%) | 64 (9.8%) | 50 (7.7%) | 54 (8.3%) | 0.338 |
| Charlson score | 3.5 (2.1) | 3.3 (1.9) | 3.4 (2.0) | 3.5 (2.1) | 3.6 (2.1) | 3.8 (2.1) | <0.001 |
| Metabolic syndrome * | 3.1 (1.2) | 2.3 (1.1) | 2.8 (1.2) | 3.3 (1.2) | 3.5 (1.1) | 3.7 (1.0) | <0.001 |
| Mean BP (mmHg) | 99.9 (13.7) | 98.4 (14.1) | 99.2 (13.3) | 100.8 (14.2) | 100.3 (13.6) | 100.7 (13.1) | 0.004 |
| Body mass index (kg/m2) | 24.6 (3.6) | 22.9 (3.5) | 24.0 (3.3) | 25.0 (3.4) | 25.4 (3.6) | 25.7 (3.6) | <0.001 |
| Waist (cm) | 87.8 (13.3) | 72.2 (10.0) | 83.2 (7.3) | 88.1 (7.6) | 92.8 (7.5) | 102.7 (10.4) | |
| Laboratory data | |||||||
| eGFR (mL/min/1.73 m2) | 24.7 (15.1) | 22.8 (15.6) | 26.1 (15.3) | 26.1 (15.1) | 26.6 (15.1) | 22.1 (13.8) | <0.001 |
| UPCR (mg/g) | 1110 (405–2542) | 1192 (447–2899) | 969 (344–2182) | 970 (377–2239) | 1107 (394–2371) | 1461 (530–3118) | <0.001 |
| Hemoglobin (g/dL) | 10.9 (2.3) | 10.5 (2.2) | 11.1 (2.3) | 11.3 (2.4) | 11.2 (2.4) | 10.6 (2.3) | <0.001 |
| Albumin (g/dL) | 3.8 (0.5) | 3.8 (0.6) | 3.9 (0.5) | 3.9 (0.5) | 3.8 (0.5) | 3.7 (0.5) | <0.001 |
| GPT (U/L) | 24.6 (24.1) | 23.8 (20.9) | 24.7 (30.0) | 26.0 (27.9) | 24.9 (21.7) | 23.8 (17.5) | 0.406 |
| Total cholesterol (mg/dL) | 191 (162–222) | 190 (160–217) | 192 (166–224) | 191 (162–223) | 192 (163–224) | 190 (160–221) | 0.609 |
| Triglyceride (mg/dL) | 126 (91–184) | 111 (81–158) | 123 (86–179) | 137 (96–194) | 131 (95–194) | 133 (98–191) | <0.001 |
| HDL-cholesterol (mg/dL) | 42.4 (13.7) | 45.2 (15.9) | 43.5 (14.0) | 42.0 (13.4) | 41.3 (12.6) | 40.3 (11.9) | <0.001 |
| LDL-cholesterol (mg/dL) | 113.1 (38.7) | 114.4 (41.3) | 113.8 (38.4) | 112.1 (37.4) | 113.8 (39.1) | 111.4 (37.3) | 0.580 |
| C-reactive protein (mg/L) | 1.2 (0.4–5.2) | 1.2 (0.4–5.1) | 0.9 (0.3–2.8) | 0.9 (0.3–3.9) | 1.2 (0.5–5.7) | 2.3 (0.5–10.5) | <0.001 |
| Sodium (mEq/L) | 138.2 (3.7) | 138.0 (3.7) | 138.3 (3.4) | 138.3 (3.4) | 138.2 (3.7) | 137.9 (4.2) | 0.014 |
| Potassium (mEq/L) | 4.4 (0.6) | 4.3 (0.6) | 4.3 (0.6) | 4.3 (0.6) | 4.4 (0.6) | 4.4 (0.6) | 0.165 |
| Calcium (mg/dL) | 9.1 (0.8) | 9.0 (0.8) | 9.1 (0.7) | 9.1 (0.8) | 9.1 (0.8) | 9.1 (0.9) | <0.001 |
| Glycosylated hemoglobin (%) | 6.5 (1.6) | 6.3 (1.5) | 6.2 (1.4) | 6.5(1.6) | 6.7(1.7) | 6.7 (1.7) | <0.001 |
| Uric acid (mg/dL) | 7.9 (1.9) | 7.8 (2.0) | 7.8 (2.0) | 7.9 (1.8) | 7.9 (1.9) | 8.0 (2.0) | 0.139 |
| Outcomes | |||||||
| ESRD | 1290 (39.5%) | 203 (31.2%) | 304 (46.6%) | 287 (44.0%) | 281 (43.0%) | 215 (32.9%) | <0.001 |
| All-cause mortality | 900 (27.6%) | 183 (28.1%) | 128 (19.6%) | 138 (21.1%) | 198 (30.3%) | 253 (38.7%) | <0.001 |
| OR for Malnutrition- Inflammation | Waist-to-Hip Ratio | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| Male | Adjusted model | 2.07 (1.38–3.12) * | 1 (reference) | 0.96 (0.64–1.43) | 0.98 (0.65–1.46) | 0.98 (0.65–1.46) |
| Adjusted model + BMI | 1.73 (1.13–2.64) * | 1 (reference) | 1.05 (0.70–1.58) | 1.06 (0.70–1.60) | 1.01 (0.66–1.56) | |
| Female | Adjusted model | 2.22 (1.39–3.54) * | 1.39 (0.89–2.18) | 1 (reference) | 1.14 (0.73–1.79) | 1.14 (0.73–1.79) |
| Adjusted model + BMI | 1.75 (1.06–2.87) * | 1.38 (0.86–2.20) | 1 (reference) | 1.16 (0.72–1.86) | 1.03 (0.63–1.69) | |
| HR for Mortality | BMI (kg/m2) | ||||||
|---|---|---|---|---|---|---|---|
| 15.0–20.0 | 20.1–22.5 | 22.6–25.0 | 25.1–27.5 | 27.6–30.0 | 30.1–35.0 | ||
| Male | Adjusted model | 2.57 (1.75–3.75) † | 1.46 (1.04–2.05) * | 1.20 (0.88–1.64) | 1.16 (0.84–1.61) | 1 (reference) | 1.35 (0.87–2.09) |
| Adjusted model + WHR | 2.59 (1.76–3.82) † | 1.52 (1.08–2.15) * | 1.23 (0.90–1.67) | 1.18 (0.85–1.64) | 1 (reference) | 1.34 (0.86–2.09) | |
| Female | Adjusted model | 1.59 (0.99–2.55) | 1.75 (1.14–2.69) * | 1.42 (0.93–2.17) | 1.33 (0.86–2.06) | 1.33 (0.83–2.14) | 1 (reference) |
| Adjusted model + WHR | 1.69 (1.04–2.73) * | 1.82 (1.18–2.81) * | 1.50 (0.98–2.29) | 1.34 (0.86–2.09) | 1.38 (0.86–2.21) | 1 (reference) | |
| HR for Mortality | Waist-to-Hip Ratio | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| Male | Adjusted model | 1.56 (1.16–2.10) * | 1 (reference) | 1.07 (0.77–1.47) | 1.28 (0.94–1.74) | 1.26 (0.94–1.69) |
| Adjusted model + BMI | 1.53 (1.13–2.05) * | 1 (reference) | 1.15 (0.84–1.60) | 1.40 (1.03–1.91) * | 1.39 (1.03–1.87) * | |
| Female | Adjusted model | 1.35 (0.94–1.95) | 1.23 (0.85–1.79) | 1 (reference) | 1.59 (1.14–2.22) * | 1.38 (0.99–1.92) |
| Adjusted model + BMI | 1.28 (0.88–1.85) | 1.21 (0.83–1.76) | 1 (reference) | 1.63 (1.17–2.27) * | 1.42 (1.02–1.99) * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, F.-C.; Chiu, Y.-W.; Kuo, M.-C.; Lin, M.-Y.; Lee, J.-J.; Hwang, S.-J.; Chang, J.-M.; Hung, C.-C.; Chen, H.-C. U-Shaped Association between Waist-to-Hip Ratio and All-Cause Mortality in Stage 3–5 Chronic Kidney Disease Patients with Body Mass Index Paradox. J. Pers. Med. 2021, 11, 1355. https://doi.org/10.3390/jpm11121355
Shen F-C, Chiu Y-W, Kuo M-C, Lin M-Y, Lee J-J, Hwang S-J, Chang J-M, Hung C-C, Chen H-C. U-Shaped Association between Waist-to-Hip Ratio and All-Cause Mortality in Stage 3–5 Chronic Kidney Disease Patients with Body Mass Index Paradox. Journal of Personalized Medicine. 2021; 11(12):1355. https://doi.org/10.3390/jpm11121355
Chicago/Turabian StyleShen, Feng-Ching, Yi-Wen Chiu, Mei-Chuan Kuo, Ming-Yen Lin, Jia-Jung Lee, Shang-Jyh Hwang, Jer-Ming Chang, Chi-Chih Hung, and Hung-Chun Chen. 2021. "U-Shaped Association between Waist-to-Hip Ratio and All-Cause Mortality in Stage 3–5 Chronic Kidney Disease Patients with Body Mass Index Paradox" Journal of Personalized Medicine 11, no. 12: 1355. https://doi.org/10.3390/jpm11121355
APA StyleShen, F. -C., Chiu, Y. -W., Kuo, M. -C., Lin, M. -Y., Lee, J. -J., Hwang, S. -J., Chang, J. -M., Hung, C. -C., & Chen, H. -C. (2021). U-Shaped Association between Waist-to-Hip Ratio and All-Cause Mortality in Stage 3–5 Chronic Kidney Disease Patients with Body Mass Index Paradox. Journal of Personalized Medicine, 11(12), 1355. https://doi.org/10.3390/jpm11121355