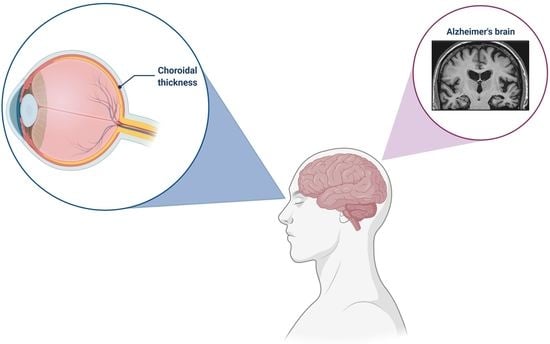
Ocular Vascular Changes: Choroidal Thickness as an Early Biomarker for Alzheimer’s Disease?
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crous-Bou, M.; Minguillón, C.; Gramunt, N.; Molinuevo, J.L. Alzheimer’s disease prevention: From risk factors to early intervention. Alzheimers Res. Ther. 2017, 9, 71. [Google Scholar] [CrossRef]
- Wong, W. Economic burden of Alzheimer disease and managed care considerations. Am. J. Manag. Care. 2020, 26 (Suppl. 8), S177–S183. [Google Scholar] [CrossRef]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, K.; Iwata, A.; Iwatsubo, T. The past, present, and future of disease-modifying therapies for Alzheimer’s disease. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017, 93, 757–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villa, C. Biomarkers for Alzheimer’s Disease: Where Do We Stand and Where Are We Going? J. Pers. Med. 2020, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.J.; Xu, W.; Ou, Y.N.; Qu, Y.; Ma, Y.H.; Huang, Y.Y.; Shen, X.N.; Chen, S.D.; Tan, L.; Zhao, Q.H.; et al. Retinal biomarkers in Alzheimer’s disease and mild cognitive impairment: A systematic review and meta-analysis. Ageing Res. Rev. 2021, 69, 101361. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M. Cerebral amyloid angiopathy: Emerging concepts. J. Stroke. 2015, 17, 17–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attems, J.; Lauda, F.; Jellinger, K.A. Unexpectedly low prevalence of intracerebral hemorrhages in sporadic cerebral amyloid angiopathy: An autopsy study. J. Neurol. 2008, 255, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Vinters, H.V. Emerging concepts in Alzheimer’s disease. Ann. Rev. Pathol. 2015, 10, 291–319. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef] [PubMed]
- London, A.; Benhar, I.; Schwartz, M. The retina as a window to the brain-from eye research to CNS disorders. Nat. Rev. Neurol. 2013, 9, 44–53. [Google Scholar] [CrossRef] [PubMed]
- An de Kreeke, J.A.; Legdeur, N.; Badissi, M.; Nguyen, H.T.; Konijnenberg, E.; Tomassen, J.; Ten Kate, M.; den Braber, A.; Maier, A.B.; Tan, H.S.; et al. Ocular biomarkers for cognitive impairment in nonagenarians; a prospective cross-sectional study. BMC Geriatr. 2020, 20, 155. [Google Scholar] [CrossRef]
- Ramírez, J.M.; Rojas, B.; Gallego, B.I.; García-Martín, E.S.; Triviño, A.; Ramírez, A.I.; Salazar, J.J.; de Hoz, R. Glia and blood retinal barrier: Effects of ocular hypertension. In Cardiovascular Disease II; iConcept Press Ltd.: Hong Kong, China, 2014; pp. 123–162. [Google Scholar]
- Moschos, M.M.; Markopoulos, I.; Chatziralli, I.; Rouvas, A.; Papageorgiou, S.G.; Ladas, I.; Vassilopoulos, D. Structural and functional impairment of the retina and optic nerve in Alzheimer’s disease. Curr. Alzheimer Res. 2012, 9, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Cabrera DeBuc, D.; Gaca-Wysocka, M.; Grzybowski, A.; Kanclerz, P. Identification of Retinal Biomarkers in Alzheimer’s Disease Using Optical Coherence Tomography: Recent Insights, Challenges, and Opportunities. J. Clin. Med. 2019, 8, 996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salobrar-Garcia, E.; Méndez-Hernández, C.; Hoz, R.D.; Ramírez, A.I.; López-Cuenca, I.; Fernández-Albarral, J.A.; Rojas, P.; Wang, S.; García-Feijoo, J.; Gil, P.; et al. Ocular Vascular Changes in Mild Alzheimer’s Disease Patients: Foveal Avascular Zone, Choroidal Thickness, and ONH Hemoglobin Analysis. J. Pers. Med. 2020, 10, 231. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Rivara, C.B.; Rocher, A.B.; Hof, P.R. The nature and effects of cortical microvascular pathology in aging and Alzheimer’s disease. Neurol. Res. 2004, 26, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, V.T. Alzheimer’s dementia begins as a disease of small blood vessels, damaged by oxidative-induced inflammation and dysregulated amyloid metabolism: Implications for early detection and therapy. FASEB J. 2011, 25, 5–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Wei, Y.; Shi, Y.; Wright, C.B.; Sun, X.; Gregori, G.; Zheng, F.; Vanner, E.A.; Lam, B.L.; Rundek, T.; et al. Altered Macular Microvasculature in Mild Cognitive Impairment and Alzheimer Disease. J. Neuroophthalmol. 2018, 38, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Golzan, S.M.; Goozee, K.; Georgevsky, D.; Avolio, A.; Chatterjee, P.; Shen, K.; Gupta, V.; Chung, R.; Savage, G.; Orr, C.F.; et al. Retinal vascular and structural changes are associated with amyloid burden in the elderly: Ophthalmic biomarkers of preclinical Alzheimer’s disease. Alzheimers Res. Ther. 2017, 9, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delaey, C.; Van De Voorde, J. Regulatory mechanisms in the retinal and choroidal circulation. Ophthalmic Res. 2000, 32, 249–256. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villa, C. Ocular Vascular Changes: Choroidal Thickness as an Early Biomarker for Alzheimer’s Disease? J. Pers. Med. 2021, 11, 1365. https://doi.org/10.3390/jpm11121365
Villa C. Ocular Vascular Changes: Choroidal Thickness as an Early Biomarker for Alzheimer’s Disease? Journal of Personalized Medicine. 2021; 11(12):1365. https://doi.org/10.3390/jpm11121365
Chicago/Turabian StyleVilla, Chiara. 2021. "Ocular Vascular Changes: Choroidal Thickness as an Early Biomarker for Alzheimer’s Disease?" Journal of Personalized Medicine 11, no. 12: 1365. https://doi.org/10.3390/jpm11121365
APA StyleVilla, C. (2021). Ocular Vascular Changes: Choroidal Thickness as an Early Biomarker for Alzheimer’s Disease? Journal of Personalized Medicine, 11(12), 1365. https://doi.org/10.3390/jpm11121365