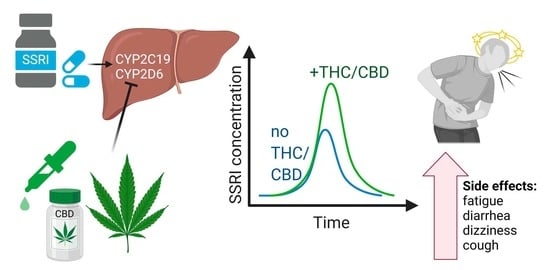
The Impact of Marijuana on Antidepressant Treatment in Adolescents: Clinical and Pharmacologic Considerations
Abstract
:1. Introduction
2. Pharmacokinetic Interactions
2.1. Cannabis and Cannabidiol Impact Cytochrome Activity
2.2. Cannabis and Cannabidiol Potentially Interact with SSRIs
2.3. Pharmacokinetics of Cannabinoids
2.4. Preliminary Models Of SSRI–Cannabinoid Pharmacokinetic Interactions
2.5. Case Illustration of SSRI-Cannabinoid Pharmacokinetic Interactions
2.6. Real-World Cannabinoid and SSRI Interactions
2.7. Data Mining of Large-Scale Clinical Effects and Hypothesis Generation Using U.S. Food and Drug Administration’s Adverse Event Reporting System (FAERS) Data
2.8. Combination Of CYP2C19-Metabolized Medications and Cannabinoids Presents Elevated Risk of Antidepressant-Related Side Effects
3. Pharmacodynamic Interactions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Merikangas, K.R.; He, J.-P.; Burstein, M.; Swanson, S.A.; Avenevoli, S.; Cui, L.; Benjet, C.; Georgiades, K.; Swendsen, J. Lifetime Prevalence of Mental Disorders in U.S. Adolescents: Results from the National Comorbidity Survey Replication—Adolescent Supplement (NCS-A). J. Am. Acad. Child. Adolesc. Psychiatry 2010, 49, 980–989. [Google Scholar] [CrossRef] [Green Version]
- Strawn, J.R.; Lu, L.; Peris, T.S.; Levine, A.; Walkup, J.T. Research Review: Pediatric anxiety disorders—What have we learnt in the last 10 years? J. Child. Psychol. Psychiatry 2021, 62, 114–139. [Google Scholar] [CrossRef]
- Mohatt, J.; Bennett, S.M.; Walkup, J.T. Treatment of Separation, Generalized, and Social Anxiety Disorders in Youths. Am. J. Psychiatry 2014, 171, 741–748. [Google Scholar] [CrossRef]
- Wehry, A.M.; Beesdo-Baum, K.; Hennelly, M.M.; Connolly, S.D.; Strawn, J.R. Assessment and Treatment of Anxiety Disorders in Children and Adolescents. Curr. Psychiatry Rep. 2015, 17, 1–11. [Google Scholar] [CrossRef]
- Brent, D.; Emslie, G.; Clarke, G.; Wagner, K.D.; Asarnow, J.R.; Keller, M.; Vitiello, B.; Ritz, L.; Iyengar, S.; Abebe, K.; et al. Switching to Another Ssri or to Venlafaxine with or without Cognitive Behavioral Therapy for Adolescents with Ssri-Resistant Depression: The Tordia Randomized Controlled Trial. JAMA 2008, 299, 901–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- March, J.S.; Vitiello, B. Clinical Messages From the Treatment for Adolescents With Depression Study (TADS). Am. J. Psychiatry 2009, 166, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Suresh, V.; Mills, J.A.; Croarkin, P.E.; Strawn, J.R. What next? A Bayesian hierarchical modeling re-examination of treatments for adolescents with selective serotonin reuptake inhibitor-resistant depression. Depress. Anxiety 2020, 37, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Walkup, J.T.; Albano, A.M.; Piacentini, J.; Birmaher, B.; Compton, S.N.; Sherrill, J.T.; Ginsburg, G.S.; Rynn, M.A.; McCracken, J.; Waslick, B.; et al. Cognitive Behavioral Therapy, Sertraline, or a Combination in Childhood Anxiety. N. Engl. J. Med. 2008, 359, 2753–2766. [Google Scholar] [CrossRef]
- Curry, J.; Rohde, P.; Simons, A.; Silva, S.; Vitiello, B.; Kratochvil, C.; Reinecke, M.; Feeny, N.; Wells, K.; Pathak, S.; et al. Predictors and Moderators of Acute Outcome in the Treatment for Adolescents With Depression Study (TADS). J. Am. Acad. Child. Adolesc. Psychiatry 2006, 45, 1427–1439. [Google Scholar] [CrossRef]
- Asselmann, E.; Wittchen, H.-U.; Lieb, R.; Beesdo-Baum, K. Sociodemographic, clinical, and functional long-term outcomes in adolescents and young adults with mental disorders. Acta Psychiatr. Scand. 2017, 137, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Kaminer, Y.; Connor, D.F.; Curry, J.F. Comorbid Adolescent Substance Use and Major Depressive Disorders: A Review. Psychiatry 2007, 4, 32–43. [Google Scholar] [PubMed]
- Strawn, J.R.; Poweleit, E.A.; Ramsey, L.B. CYP2C19-Guided Escitalopram and Sertraline Dosing in Pediatric Patients: A Pharmacokinetic Modeling Study. J. Child. Adolesc. Psychopharmacol. 2019, 29, 340–347. [Google Scholar] [CrossRef] [Green Version]
- Weinberger, A.H.; Zhu, J.; Lee, J.; Anastasiou, E.; Copeland, J.; Goodwin, R.D. Cannabis use among youth in the United States, 2004–2016: Faster rate of increase among youth with depression. Drug Alcohol Depend. 2020, 209, 107894. [Google Scholar] [CrossRef]
- Caudle, K.E.; Dunnenberger, H.M.; Freimuth, R.; Peterson, J.F.; Burlison, J.D.; Whirl-Carrillo, M.; Scott, S.A.; Rehm, H.L.; Williams, M.S.; Klein, T.E.; et al. Standardizing terms for clinical pharmacogenetic test results: Consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC). Genet. Med. 2017, 19, 215–223. [Google Scholar] [CrossRef]
- Hicks, J.K.; Bishop, J.R.; Sangkuhl, K.; Müller, D.J.; Ji, Y.; Leckband, S.G.; Leeder, J.S.; Graham, R.L.; Chiulli, D.L.; Llerena, A.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline forCYP2D6andCYP2C19Genotypes and Dosing of Selective Serotonin Reuptake Inhibitors. Clin. Pharmacol. Ther. 2015, 98, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Strom, C.M.; Goos, D.; Crossley, B.; Zhang, K.; Buller-Burkle, A.; Jarvis, M.; Quan, F.; Peng, M.; Sun, W. Testing for Variants in Cyp2c19: Population Frequencies and Testing Experience in a Clinical Laboratory. Genet. Med. 2012, 14, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Aldrich, S.L.; Poweleit, E.A.; Prows, C.A.; Martin, L.J.; Strawn, J.R.; Ramsey, L.B. Influence of CYP2C19 Metabolizer Status on Escitalopram/Citalopram Tolerability and Response in Youth With Anxiety and Depressive Disorders. Front. Pharmacol. 2019, 10, 99. [Google Scholar] [CrossRef]
- Strawn, J.R.; Mills, J.A.; Schroeder, H.; Mossman, S.A.; Varney, S.T.; Ramsey, L.B.; Poweleit, E.A.; Desta, Z.; Cecil, K.; DelBello, M.P. Escitalopram in Adolescents with Generalized Anxiety Disorder: A Double-Blind, Randomized, Placebo-Controlled Study. J. Clin. Psychiatry 2020, 81. [Google Scholar] [CrossRef] [PubMed]
- Poweleit, E.A.; Aldrich, S.L.; Martin, L.J.; Hahn, D.; Strawn, J.R.; Ramsey, L.B. Pharmacogenetics of Sertraline Tolerability and Response in Pediatric Anxiety and Depressive Disorders. J. Child. Adolesc. Psychopharmacol. 2019, 29, 348–361. [Google Scholar] [CrossRef]
- Strawn, J.R.; Mills, J.A.; Sauley, B.A.; Welge, J.A. The Impact of Antidepressant Dose and Class on Treatment Response in Pediatric Anxiety Disorders: A Meta-Analysis. J. Am. Acad. Child. Adolesc. Psychiatry 2018, 57, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Rossow, K.M.; Aka, I.T.; Maxwell-Horn, A.C.; Roden, D.M.; Van Driest, S.L. Pharmacogenetics to Predict Adverse Events Associated With Antidepressants. Pediatrics 2020, 146, e20200957. [Google Scholar] [CrossRef] [PubMed]
- Sakolsky, D.J.; Perel, J.M.; Emslie, G.J.; Clarke, G.N.; Wagner, K.D.; Vitiello, B.; Keller, M.B.; Birmaher, B.; Asarnow, J.R.; Ryan, N.D.; et al. Antidepressant Exposure as a Predictor of Clinical Outcomes in the Treatment of Resistant Depression in Adolescents (TORDIA) Study. J. Clin. Psychopharmacol. 2011, 31, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Howlett, A.C. The cannabinoid receptors. Prostaglandins Other Lipid Mediat. 2002, 68, 619–631. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis Sativa L. Prog. Chem. Org. Nat. Prod. 2017, 103, 1–36. [Google Scholar]
- Fischedick, J.; Van Der Kooy, F.; Verpoorte, R. Cannabinoid Receptor 1 Binding Activity and Quantitative Analysis of Cannabis sativa L. Smoke and Vapor. Chem. Pharm. Bull. 2010, 58, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, K.; Yamaori, S.; Funahashi, T.; Kimura, T.; Yamamoto, I. Cytochrome P450 enzymes involved in the metabolism of tetrahydrocannabinols and cannabinol by human hepatic microsomes. Life Sci. 2007, 80, 1415–1419. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Yamaori, S.; Takeda, S.; Yamamoto, I.; Watanabe, K. Identification of cytochrome P450 enzymes responsible for metabolism of cannabidiol by human liver microsomes. Life Sci. 2011, 89, 165–170. [Google Scholar] [CrossRef]
- Yamaori, S.; Maeda, C.; Yamamoto, I.; Watanabe, K. Differential inhibition of human cytochrome P450 2A6 and 2B6 by major phytocannabinoids. Forensic Toxicol. 2011, 29, 117–124. [Google Scholar] [CrossRef]
- Jiang, R.; Yamaori, S.; Okamoto, Y.; Yamamoto, I.; Watanabe, K. Cannabidiol Is a Potent Inhibitor of the Catalytic Activity of Cytochrome P450 2C19. Drug Metab. Pharmacokinet. 2013, 28, 332–338. [Google Scholar] [CrossRef] [Green Version]
- Zendulka, O.; Dovrtělová, G.; Nosková, K.; Turjap, M.; Šulcová, A.; Hanuš, L.; Juřica, J. Cannabinoids and Cytochrome P450 Interactions. Curr. Drug Metab. 2016, 17, 206–226. [Google Scholar] [CrossRef]
- Bansal, S.; Maharao, N.; Paine, M.F.; Unadkat, J.D. Predicting the Potential for Cannabinoids to Precipitate Pharmacokinetic Drug Interactions via Reversible Inhibition or Inactivation of Major Cytochromes P450. Drug Metab. Dispos. 2020, 48, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Giraud, C.; Tran, A.; Rey, E.; Vincent, J.; Tréluyer, J.-M.; Pons, G. In vitro characterization of clobazam metabolism by recombinant cytochrome P450 enzymes: Importance of CYP2C19. Drug Metab. Dispos. 2004, 32, 1279–1286. [Google Scholar] [CrossRef] [Green Version]
- Contin, M.; Sangiorgi, S.; Riva, R.; Parmeggiani, A.; Albani, F.; Baruzzi, A. Evidence of Polymorphic CYP2C19 Involvement in the Human Metabolism of N-Desmethylclobazam. Ther. Drug Monit. 2002, 24, 737–741. [Google Scholar] [CrossRef]
- Kosaki, K.; Tamura, K.; Sato, R.; Samejima, H.; Tanigawara, Y.; Takahashi, T. A Major Influence of Cyp2c19 Genotype on the Steady-State Concentration of N-Desmethylclobazam. Brain Dev. 2004, 26, 530–534. [Google Scholar] [CrossRef]
- Qian, Y.; Gurley, B.J.; Markowitz, J.S. The Potential for Pharmacokinetic Interactions Between Cannabis Products and Conventional Medications. J. Clin. Psychopharmacol. 2019, 39, 462–471. [Google Scholar] [CrossRef]
- Seo, T.; Nagata, R.; Ishitsu, T.; Murata, T.; Takaishi, C.; Hori, M.; Nakagawa, K. Impact ofCYP2C19polymorphisms on the efficacy of clobazam therapy. Pharmacogenomics 2008, 9, 527–537. [Google Scholar] [CrossRef]
- Geffrey, A.L.; Pollack, S.F.; Bruno, P.L.; Thiele, E.A. Drug-drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia 2015, 56, 1246–1251. [Google Scholar] [CrossRef]
- Patsalos, P.N.; Szaflarski, J.P.; Gidal, B.; VanLandingham, K.; Critchley, D.; Morrison, G. Clinical implications of trials investigating drug-drug interactions between cannabidiol and enzyme inducers or inhibitors or common antiseizure drugs. Epilepsia 2020, 61, 1854–1868. [Google Scholar] [CrossRef]
- Al Maruf, A.; Greenslade, A.; Arnold, P.D.; Bousman, C. Antidepressant pharmacogenetics in children and young adults: A systematic review. J. Affect. Disord. 2019, 254, 98–108. [Google Scholar] [CrossRef]
- Grotenhermen, F. Pharmacokinetics and Pharmacodynamics of Cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360. [Google Scholar] [CrossRef]
- Agurell, S.; Halldin, M.; Lindgren, J.E.; Ohlsson, A.; Widman, M.; Gillespie, H.; Hollister, L. Pharmacokinetics and metabolism of delta 1-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacol. Rev. 1986, 38, 21–43. [Google Scholar]
- Strougo, A.; Zuurman, L.; Roy, C.; Pinquier, J.; Van Gerven, J.; Cohen, A.; Schoemaker, R. Modelling of the concentration—Effect relationship of THC on central nervous system parameters and heart rate—Insight into its mechanisms of action and a tool for clinical research and development of cannabinoids. J. Psychopharmacol. 2008, 22, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Sempio, C.; Huestis, M.A.; Mikulich-Gilbertson, S.K.; Klawitter, J.; Christians, U.; Henthorn, T.K. Population Pharmacokinetic Modeling of Plasma Delta9-Tetrahydrocannabinol and an Active and Inactive Metabolite Following Controlled Smoked Cannabis Administration. Br. J. Clin. Pharmacol. 2020, 86, 611–619. [Google Scholar] [CrossRef]
- Proost, J.H.; Meijer, D.K. MW/Pharm, an integrated software package for drug dosage regimen calculation and therapeutic drug monitoring. Comput. Biol. Med. 1992, 22, 155–163. [Google Scholar] [CrossRef]
- Morrison, G.; Crockett, J.; Blakey, G.; Sommerville, K. A Phase 1, Open-Label, Pharmacokinetic Trial to Investigate Possible Drug-Drug Interactions Between Clobazam, Stiripentol, or Valproate and Cannabidiol in Healthy Subjects. Clin. Pharmacol. Drug Dev. 2019, 8, 1009–1031. [Google Scholar] [CrossRef]
- Hiemke, C.; Bergemann, N.; Clement, H.W.; Conca, A.; Deckert, J.; Domschke, K.; Eckermann, G.; Egberts, K.; Gerlach, M.; Greiner, C.; et al. Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology: Update 2017. Pharmacopsychiatry 2018, 51, 9–62. [Google Scholar] [CrossRef] [Green Version]
- Foster, B.C.; Abramovici, H.; Harris, C.S. Cannabis and Cannabinoids: Kinetics and Interactions. Am. J. Med. 2019, 132, 1266–1270. [Google Scholar] [CrossRef] [PubMed]
- Iacopetti, C.L.; Packer, C.D. Cannabinoid Hyperemesis Syndrome: A Case Report and Review of Pathophysiology. Clin. Med. Res. 2014, 12, 65–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarangdhar, M.; Tabar, S.; Schmidt, C.; Kushwaha, A.; Shah, K.; Dahlquist, J.E.; Jegga, A.G.; Aronow, B.J. Data mining differential clinical outcomes associated with drug regimens using adverse event reporting data. Nat. Biotechnol. 2016, 34, 697–700. [Google Scholar] [CrossRef]
- Lexicomp Online. Lexi-Drugs; Lexi-Comp, Inc.: Hudson, OH, USA, 2016; Available online: http://webstore.lexi.com/ONLINE (accessed on 8 April 2021).
- Norén, G.N.; Hopstadius, J.; Bate, A. Shrinkage observed-to-expected ratios for robust and transparent large-scale pattern discovery. Stat. Methods Med. Res. 2011, 22, 57–69. [Google Scholar] [CrossRef]
- Norén, G.N.; Sundberg, R.; Bate, A.; Edwards, I.R. A statistical methodology for drug–drug interaction surveillance. Stat. Med. 2008, 27, 3057–3070. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Yekutieli, D. The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 2001, 29, 1165–1188. [Google Scholar] [CrossRef]
- Takahashi, E.; Katayama, M.; Niimi, K.; Itakura, C. Additive subthreshold dose effects of cannabinoid CB1 receptor antagonist and selective serotonin reuptake inhibitor in antidepressant behavioral tests. Eur. J. Pharmacol. 2008, 589, 149–156. [Google Scholar] [CrossRef]
- Ortega, J.E.; Gonzalez-Lira, V.; Horrillo, I.; Herrera-Marschitz, M.; Callado, L.F.; Meana, J.J. Additive effect of rimonabant and citalopram on extracellular serotonin levels monitored with in vivo microdialysis in rat brain. Eur. J. Pharmacol. 2013, 709, 13–19. [Google Scholar] [CrossRef]
- Hill, M.N.; Sun, J.C.; Tse, M.T.L.; Gorzalka, B.B. Altered responsiveness of serotonin receptor subtypes following long-term cannabinoid treatment. Int. J. Neuropsychopharmacol. 2005, 9, 277–286. [Google Scholar] [CrossRef]
- Franklin, J.M.; Carrasco, G.A. Cannabinoid Receptor Agonists Upregulate and Enhance Serotonin 2a (5-Ht(2a)) Receptor Activity Via Erk1/2 Signaling. Synapse 2013, 67, 145–159. [Google Scholar] [CrossRef] [Green Version]
- Best, A.R.; Regehr, W.G. Serotonin Evokes Endocannabinoid Release and Retrogradely Suppresses Excitatory Synapses. J. Neurosci. 2008, 28, 6508–6515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darmani, N.A.; Janoyan, J.J.; Crim, J.; Ramirez, J. Receptor mechanism and antiemetic activity of structurally-diverse cannabinoids against radiation-induced emesis in the least shrew. Eur. J. Pharmacol. 2007, 563, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Pertwee, R.G. The Diverse Cb1 and Cb2 Receptor Pharmacology of Three Plant Cannabinoids: Delta9-Tetrahydrocannabinol, Cannabidiol and Delta9-Tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef] [Green Version]
- Hales, C.M.; Kit, B.K.; Gu, Q.; Ogden, C.L. Trends in Prescription Medication Use Among Children and Adolescents—United States, 1999–2014. JAMA 2018, 319, 2009–2020. [Google Scholar] [CrossRef]
- Cox, E.J.; Maharao, N.; Patilea-Vrana, G.; Unadkat, J.D.; Rettie, A.E.; McCune, J.S.; Paine, M.F. A marijuana-drug interaction primer: Precipitants, pharmacology, and pharmacokinetics. Pharmacol. Ther. 2019, 201, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Luft, M.J.; Lamy, M.; DelBello, M.P.; McNamara, R.K.; Strawn, J.R. Antidepressant-Induced Activation in Children and Adolescents: Risk, Recognition and Management. Curr. Probl. Pediatr. Adolesc. Heal. Care 2018, 48, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Jakubovski, E.; Varigonda, A.L.; Freemantle, N.; Taylor, M.J.; Bloch, M.H. Systematic Review and Meta-Analysis: Dose-Response Relationship of Selective Serotonin Reuptake Inhibitors in Major Depressive Disorder. Am. J. Psychiatry 2016, 173, 174–183. [Google Scholar] [CrossRef]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S. Cannabinoids for Medical Use: A Systematic Review and Meta-Analysis. JAMA J. Am. Med. Assoc. 2015, 313, 2456–2473. [Google Scholar] [CrossRef] [PubMed]





| IC50µ (µM) 1 | ||
|---|---|---|
| CBD | THC | |
| CYP2C19 | 0.30 ± 0.03 | 0.57 ± 0.22 |
| CYP2D6 | 0.95 ± 0.50 | 1.28 ± 0.25 |
| CYP3A4 | 0.38 ± 0.11 | 1.30 ± 0.34 |
| THC or Low-Dose CBD 1 | ||
|---|---|---|
| − | + | |
| t1/2 (h) | 21.3 | 28.3 |
| AUC24, 10 mg q.d. (days ng/mL) | 17.1 | 23.1 |
| Cmax, 10 mg q.d. (ng/mL) | 22.7 | 28.4 |
| AUC24, 20 mg q.d. (days ng/mL) | 34.1 | 46.1 |
| Cmax, 20 mg q.d. (ng/mL) | 45.4 | 56.9 |
| THC or Low-Dose CBD | ||
|---|---|---|
| − | + | |
| t1/2 (h) | 22.1 | 29.5 |
| AUC24, 50 mg q.d. (days ng/mL) | 18.0 | 23.7 |
| Cmax, 50 mg q.d. (ng/mL) | 21.9 | 27.4 |
| AUC24, 100 mg q.d. (days ng/mL) | 36.1 | 47.9 |
| Cmax, 100 mg q.d. (ng/mL) | 44.0 | 55.3 |
| AUC24, 150 mg q.d. (days ng/mL) | 54.2 | 72.1 |
| Cmax, 150 mg q.d. (ng/mL) | 66.0 | 83.2 |
| AUC24, 200mg q.d. (days ng/mL) | 72.3 | 98.45 |
| Cmax, 200 mg q.d. (ng/mL) | 88.0 | 111.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaughn, S.E.; Strawn, J.R.; Poweleit, E.A.; Sarangdhar, M.; Ramsey, L.B. The Impact of Marijuana on Antidepressant Treatment in Adolescents: Clinical and Pharmacologic Considerations. J. Pers. Med. 2021, 11, 615. https://doi.org/10.3390/jpm11070615
Vaughn SE, Strawn JR, Poweleit EA, Sarangdhar M, Ramsey LB. The Impact of Marijuana on Antidepressant Treatment in Adolescents: Clinical and Pharmacologic Considerations. Journal of Personalized Medicine. 2021; 11(7):615. https://doi.org/10.3390/jpm11070615
Chicago/Turabian StyleVaughn, Samuel E., Jeffrey R. Strawn, Ethan A. Poweleit, Mayur Sarangdhar, and Laura B. Ramsey. 2021. "The Impact of Marijuana on Antidepressant Treatment in Adolescents: Clinical and Pharmacologic Considerations" Journal of Personalized Medicine 11, no. 7: 615. https://doi.org/10.3390/jpm11070615
APA StyleVaughn, S. E., Strawn, J. R., Poweleit, E. A., Sarangdhar, M., & Ramsey, L. B. (2021). The Impact of Marijuana on Antidepressant Treatment in Adolescents: Clinical and Pharmacologic Considerations. Journal of Personalized Medicine, 11(7), 615. https://doi.org/10.3390/jpm11070615