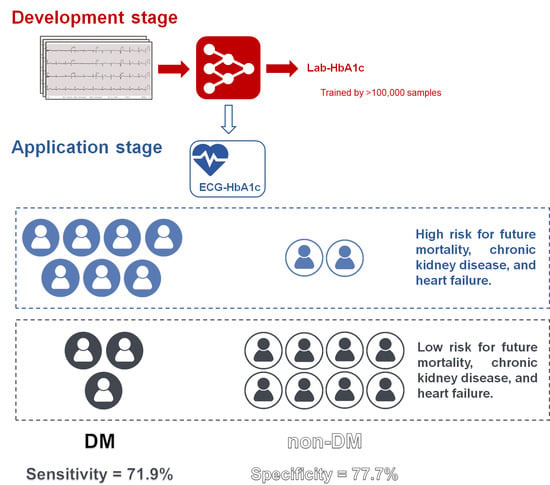
Deep Learning Algorithm for Management of Diabetes Mellitus via Electrocardiogram-Based Glycated Hemoglobin (ECG-HbA1c): A Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Population
2.2. Observational Variables
2.3. Implementation of the Deep Learning Model
2.4. Statistical Analysis and Model Performance Assessment
3. Results
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the international diabetes federation diabetes atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- John, W.G.; Hillson, R.; Alberti, S.G. Use of haemoglobin A1c (HbA1c) in the diagnosis of diabetes mellitus. The implementation of World Health Organisation (WHO) guidance 2011. Pract. Diabetes 2012, 29, 12-12a. [Google Scholar] [CrossRef]
- Lindström, J.; Tuomilehto, J. The diabetes risk score: A practical tool to predict type 2 diabetes risk. Diabetes Care 2003, 26, 725–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.; Simmons, R.K.; Harding, A.H.; Wareham, N.J.; Griffin, S.J. A simple risk score identifies individuals at high risk of developing Type 2 diabetes: A prospective cohort study. Fam. Pract. 2008, 25, 191–196. [Google Scholar] [CrossRef] [Green Version]
- Hippisley-Cox, J.; Coupland, C.; Robson, J.; Sheikh, A.; Brindle, P. Predicting risk of type 2 diabetes in England and Wales: Prospective derivation and validation of QDScore. BMJ 2009, 338, b880. [Google Scholar] [CrossRef] [Green Version]
- Noble, D.; Mathur, R.; Dent, T.; Meads, C.; Greenhalgh, T. Risk models and scores for type 2 diabetes: Systematic review. BMJ 2011, 343, d7163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, A.N.; Dagogo-Jack, S. Comorbidities of diabetes and hypertension: Mechanisms and approach to target organ protection. J. Clin. Hypertens. 2011, 13, 244–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jelinek, H.F.; Osman, W.M.; Khandoker, A.H.; Khalaf, K.; Lee, S.; Almahmeed, W.; Alsafar, H.S. Clinical profiles, comorbidities and complications of type 2 diabetes mellitus in patients from United Arab Emirates. BMJ Open Diabetes Res. Care 2017, 5, e000427. [Google Scholar] [CrossRef] [Green Version]
- Htay, T.; Soe, K.; Lopez-Perez, A.; Doan, A.H.; Romagosa, M.A.; Aung, K. Mortality and Cardiovascular Disease in Type 1 and Type 2 Diabetes. Curr. Cardiol. Rep. 2019, 21, 45. [Google Scholar] [CrossRef] [PubMed]
- Standards of medical care in diabetes-2015 abridged for primary care providers. Clin. Diabetes Publ. Am. Diabetes Assoc. 2015, 33, 97–111. [CrossRef] [Green Version]
- Sabanayagam, C.; Liew, G.; Tai, E.S.; Shankar, A.; Lim, S.C.; Subramaniam, T.; Wong, T.Y. Relationship between glycated haemoglobin and microvascular complications: Is there a natural cut-off point for the diagnosis of diabetes? Diabetologia 2009, 52, 1279–1289. [Google Scholar] [CrossRef] [Green Version]
- El-Salem, K.; Ammari, F.; Khader, Y.; Dhaimat, O. Elevated glycosylated hemoglobin is associated with subclinical neuropathy in neurologically asymptomatic diabetic patients: A prospective study. J. Clin. Neurophysiol. 2009, 26, 50–53. [Google Scholar] [CrossRef]
- Vos, F.E.; Schollum, J.B.; Walker, R.J. Glycated albumin is the preferred marker for assessing glycaemic control in advanced chronic kidney disease. NDT Plus 2011, 4, 368–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khaw, K.T.; Wareham, N.; Bingham, S.; Luben, R.; Welch, A.; Day, N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: The European prospective investigation into cancer in Norfolk. Ann. Intern. Med. 2004, 141, 413–420. [Google Scholar] [CrossRef]
- Chowdhury, M.; Goonan, K.; Peacock, S.; Simpson, R. On Missing Values of HbA1c in Diabetes Quality of Care Evaluation. Diabetes 2001, 50 (Suppl. 2). [Google Scholar]
- Murdoch, T.B.; Detsky, A.S. The inevitable application of big data to health care. JAMA 2013, 309, 1351–1352. [Google Scholar] [CrossRef]
- Yeboah, J.; McClelland, R.L.; Polonsky, T.S.; Burke, G.L.; Sibley, C.T.; O’Leary, D.; Carr, J.J.; Goff, D.C.; Greenland, P.; Herrington, D.M. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA 2012, 308, 788–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diller, G.P.; Kempny, A.; Babu-Narayan, S.V.; Henrichs, M.; Brida, M.; Uebing, A.; Lammers, A.E.; Baumgartner, H.; Li, W.; Wort, S.J.; et al. Machine learning algorithms estimating prognosis and guiding therapy in adult congenital heart disease: Data from a single tertiary centre including 10,019 patients. Eur. Heart J. 2019, 40, 1069–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.C.; Lin, C.S.; Tsai, C.S.; Tsao, T.P.; Cheng, C.C.; Liou, J.T.; Lin, W.S.; Cheng, S.M.; Lou, Y.S.; Lee, C.C.; et al. A Deep-Learning Algorithm for Detecting Acute Myocardial Infarction. EuroIntervention J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2021. [Google Scholar] [CrossRef]
- Chang, D.-W.; Lin, C.-S.; Tsao, T.-P.; Lee, C.-C.; Chen, J.-T.; Tsai, C.-S.; Lin, W.-S.; Lin, C. Detecting Digoxin Toxicity by Artificial Intelligence-Assisted Electrocardiography. Int. J. Environ. Res. Public Health 2021, 18, 3839. [Google Scholar] [CrossRef] [PubMed]
- Attia, Z.I.; Noseworthy, P.A.; Lopez-Jimenez, F.; Asirvatham, S.J.; Deshmukh, A.J.; Gersh, B.J.; Carter, R.E.; Yao, X.; Rabinstein, A.A.; Erickson, B.J.; et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: A retrospective analysis of outcome prediction. Lancet 2019, 394, 861–867. [Google Scholar] [CrossRef]
- Hannun, A.Y.; Rajpurkar, P.; Haghpanahi, M.; Tison, G.H.; Bourn, C.; Turakhia, M.P.; Ng, A.Y. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat. Med. 2019, 25, 65–69. [Google Scholar] [CrossRef]
- Galloway, C.D.; Valys, A.V.; Shreibati, J.B.; Treiman, D.L.; Petterson, F.L.; Gundotra, V.P.; Albert, D.E.; Attia, Z.I.; Carter, R.E.; Asirvatham, S.J.; et al. Development and validation of a deep-learning model to screen for hyperkalemia from the electrocardiogram. JAMA Cardiol. 2019, 4, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S.; Lin, C.; Fang, W.H.; Hsu, C.J.; Chen, S.J.; Huang, K.H.; Lin, W.S.; Tsai, C.S.; Kuo, C.C.; Chau, T.; et al. A deep-learning algorithm (ECG12Net) for detecting hypokalemia and hyperkalemia by electrocardiography: Algorithm development. JMIR Med. Inform. 2020, 8, e15931. [Google Scholar] [CrossRef] [PubMed]
- Attia, Z.I.; Kapa, S.; Lopez-Jimenez, F.; McKie, P.M.; Ladewig, D.J.; Satam, G.; Pellikka, P.A.; Enriquez-Sarano, M.; Noseworthy, P.A.; Munger, T.M.; et al. Screening for cardiac contractile dysfunction using an artificial intelligence-enabled electrocardiogram. Nat. Med. 2019, 25, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Attia, Z.I.; Kapa, S.; Yao, X.; Lopez-Jimenez, F.; Mohan, T.L.; Pellikka, P.A.; Carter, R.E.; Shah, N.D.; Friedman, P.A.; Noseworthy, P.A. Prospective validation of a deep learning electrocardiogram algorithm for the detection of left ventricular systolic dysfunction. J. Cardiovasc. Electrophysiol. 2019, 30, 668–674. [Google Scholar] [CrossRef]
- Kwon, J.M.; Kim, K.H.; Akkus, Z.; Jeon, K.H.; Park, J.; Oh, B.H. Artificial intelligence for detecting mitral regurgitation using electrocardiography. J. Electrocardiol. 2020, 59, 151–157. [Google Scholar] [CrossRef]
- Kwon, J.M.; Lee, S.Y.; Jeon, K.H.; Lee, Y.; Kim, K.H.; Park, J.; Oh, B.H.; Lee, M.M. Deep learning-based algorithm for detecting aortic stenosis using electrocardiography. J. Am. Heart Assoc. 2020, 9, e014717. [Google Scholar] [CrossRef]
- Porumb, M.; Stranges, S.; Pescape, A.; Pecchia, L. Precision medicine and artificial intelligence: A pilot study on deep learning for hypoglycemic events detection based on ECG. Sci. Rep. 2020, 10, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attia, Z.I.; Friedman, P.A.; Noseworthy, P.A.; Lopez-Jimenez, F.; Ladewig, D.J.; Satam, G.; Pellikka, P.A.; Munger, T.M.; Asirvatham, S.J.; Scott, C.G.; et al. Age and sex estimation using artificial intelligence from standard 12-lead ECGs. Circulation. Arrhythmia Electrophysiol. 2019, 12, e007284. [Google Scholar] [CrossRef]
- Nakagami, T.; Tajima, N.; Oizumi, T.; Karasawa, S.; Wada, K.; Kameda, W.; Susa, S.; Kato, T.; Daimon, M. Hemoglobin A1c in predicting progression to diabetes. Diabetes Res. Clin. Pract. 2010, 87, 126–131. [Google Scholar] [CrossRef]
- Buuren, S.v.; Groothuis-Oudshoorn, K. Mice: Multivariate imputation by chained equations in R. J. Stat. Softw. 2010, 45, 1–67. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41, S55–S64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.; Lin, C.-S.; Lee, D.-J.; Lee, C.-C.; Chen, S.-J.; Tsai, S.-H.; Kuo, F.-C.; Chau, T.; Lin, S.-H. Artificial intelligence assisted electrocardiography for early diagnosis of thyrotoxic periodic paralysis. J. Endocr. Soc. 2021, 5, bvab120. [Google Scholar] [CrossRef]
- Lin, C.; Hsu, C.J.; Lou, Y.S.; Yeh, S.J.; Lee, C.C.; Su, S.L.; Chen, H.C. Artificial Intelligence Learning Semantics via External Resources for Classifying Diagnosis Codes in Discharge Notes. J. Med. Internet Res. 2017, 19, e380. [Google Scholar] [CrossRef] [Green Version]
- Simpson, A.J. Over-sampling in a deep neural network. arXiv 2015, arXiv:1502.03648. [Google Scholar]
- Axelsen, L.N.; Calloe, K.; Braunstein, T.H.; Riemann, M.; Hofgaard, J.P.; Liang, B.; Jensen, C.F.; Olsen, K.B.; Bartels, E.D.; Baandrup, U.; et al. Diet-induced pre-diabetes slows cardiac conductance and promotes arrhythmogenesis. Cardiovasc. Diabetol. 2015, 14, 87. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Zhang, W.; Zhu, L.; Lin, N.; Niu, Y.; Li, X.; Lu, S.; Zhang, H.; Wang, X.; Wen, J.; et al. Resting heart rate and impaired glucose regulation in middle-aged and elderly Chinese people: A cross-sectional analysis. BMC Cardiovasc. Disord. 2017, 17, 246. [Google Scholar] [CrossRef] [Green Version]
- Gudul, N.E.; Karabag, T.; Sayin, M.R.; Bayraktaroglu, T.; Aydin, M. Atrial conduction times and left atrial mechanical functions and their relation with diastolic function in prediabetic patients. Korean J. Intern. Med. 2017, 32, 286–294. [Google Scholar] [CrossRef]
- Wang, L.; Cui, L.; Wang, Y.; Vaidya, A.; Chen, S.; Zhang, C.; Zhu, Y.; Li, D.; Hu, F.B.; Wu, S.; et al. Resting heart rate and the risk of developing impaired fasting glucose and diabetes: The Kailuan prospective study. Int. J. Epidemiol. 2015, 44, 689–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poplin, R.; Varadarajan, A.V.; Blumer, K.; Liu, Y.; McConnell, M.V.; Corrado, G.S.; Peng, L.; Webster, D.R. Prediction of cardiovascular risk factors from retinal fundus photographs via deep learning. Nat. Biomed. Eng. 2018, 2, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Mu, Y.; Zhao, J.; Wang, X.; Che, H. IGRNet: A Deep Learning Model for Non-Invasive, Real-Time Diagnosis of Prediabetes through Electrocardiograms. Sensors 2020, 20, 2556. [Google Scholar] [CrossRef]
- Hirst, J.A.; McLellan, J.H.; Price, C.P.; English, E.; Feakins, B.G.; Stevens, R.J.; Farmer, A.J. Performance of point-of-care HbA1c test devices: Implications for use in clinical practice—A systematic review and meta-analysis. Clin. Chem. Lab. Med. 2017, 55, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Hira, R.S.; Kennedy, K.; Nambi, V.; Jneid, H.; Alam, M.; Basra, S.S.; Ho, P.M.; Deswal, A.; Ballantyne, C.M.; Petersen, L.A.; et al. Frequency and practice-level variation in inappropriate aspirin use for the primary prevention of cardiovascular disease: Insights from the National Cardiovascular Disease Registry’s Practice Innovation and Clinical Excellence registry. J. Am. Coll. Cardiol. 2015, 65, 111–121. [Google Scholar] [CrossRef] [Green Version]
- Cooney, M.T.; Dudina, A.; De Bacquer, D.; Fitzgerald, A.; Conroy, R.; Sans, S.; Menotti, A.; De Backer, G.; Jousilahti, P.; Keil, U.; et al. How much does HDL cholesterol add to risk estimation? A report from the SCORE Investigators. Eur. J. Cardiovasc. Prev. Rehabil. 2009, 16, 304–314. [Google Scholar] [CrossRef]
- Dudina, A.; Cooney, M.T.; Bacquer, D.D.; Backer, G.D.; Ducimetière, P.; Jousilahti, P.; Keil, U.; Menotti, A.; Njølstad, I.; Oganov, R.; et al. Relationships between body mass index, cardiovascular mortality, and risk factors: A report from the SCORE investigators. Eur. J. Cardiovasc. Prev. Rehabil. 2011, 18, 731–742. [Google Scholar] [CrossRef]
- Pani, L.N.; Korenda, L.; Meigs, J.B.; Driver, C.; Chamany, S.; Fox, C.S.; Sullivan, L.; D’Agostino, R.B.; Nathan, D.M. Effect of aging on A1C levels in individuals without diabetes: Evidence from the Framingham Offspring Study and the National Health and Nutrition Examination Survey 2001–2004. Diabetes Care 2008, 31, 1991–1996. [Google Scholar] [CrossRef] [Green Version]
- Dubowitz, N.; Xue, W.; Long, Q.; Ownby, J.G.; Olson, D.E.; Barb, D.; Rhee, M.K.; Mohan, A.V.; Watson-Williams, P.I.; Jackson, S.L.; et al. Aging is associated with increased HbA1c levels, independently of glucose levels and insulin resistance, and also with decreased HbA1c diagnostic specificity. Diabet. Med. 2014, 31, 927–935. [Google Scholar] [CrossRef]
- Masuch, A.; Friedrich, N.; Roth, J.; Nauck, M.; Müller, U.A.; Petersmann, A. Preventing misdiagnosis of diabetes in the elderly: Age-dependent HbA1c reference intervals derived from two population-based study cohorts. BMC Endocr. Disord. 2019, 19, 20. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.; Lou, Y.S.; Tsai, D.J.; Lee, C.C.; Hsu, C.J.; Wu, D.C.; Wang, M.C.; Fang, W.H. Projection Word Embedding Model With Hybrid Sampling Training for Classifying ICD-10-CM Codes: Longitudinal Observational Study. JMIR Med. Inform. 2019, 7, e14499. [Google Scholar] [CrossRef] [PubMed]
- Legato, M.J.; Gelzer, A.; Goland, R.; Ebner, S.A.; Rajan, S.; Villagra, V.; Kosowski, M. Gender-specific care of the patient with diabetes: Review and recommendations. Gend. Med. 2006, 3, 131–158. [Google Scholar] [CrossRef]
- Dong, W.; Wan, E.Y.F.; Bedford, L.E.; Wu, T.; Wong, C.K.H.; Tang, E.H.M.; Lam, C.L.K. Prediction models for the risk of cardiovascular diseases in Chinese patients with type 2 diabetes mellitus: A systematic review. Public Health 2020, 186, 144–156. [Google Scholar] [CrossRef] [PubMed]
- VanderWeele, T.J.; Shpitser, I. On the definition of a confounder. Ann. Stat. 2013, 41, 196–220. [Google Scholar] [CrossRef] [PubMed]
- Noseworthy, P.A.; Attia, Z.I.; Brewer, L.C.; Hayes, S.N.; Yao, X.; Kapa, S.; Friedman, P.A.; Lopez-Jimenez, F. Assessing and mitigating bias in medical artificial intelligence: The effects of race and ethnicity on a deep learning model for ECG analysis. Circ. Arrhythmia Electrophysiol. 2020, 13, e007988. [Google Scholar] [CrossRef]
- Castelvecchi, D. Can we open the black box of AI? Nature 2016, 538, 20–23. [Google Scholar] [CrossRef] [Green Version]






| Development Cohort (N/n = 57,185/104,823) | Validation Cohort (N/n = 1539/2190) | Follow-Up Cohort (N/n = 3293/3293) | p-Value | |
|---|---|---|---|---|
| Location | <0.001 | |||
| OPD/HEC | 56,511 (53.9%) | 2190 (100.0%) | 3293 (100.0%) | |
| IPD/EMR | 48,312 (46.1%) | 0 (0.0%) | 0 (0.0%) | |
| Gender (Male) | 59182 (56.5%) | 1124 (51.3%) | 1746 (53.0%) | <0.001 |
| Age (years) | 60.9 ± 17.1 | 56.0 ± 14.8 | 58.8 ± 15.0 | <0.001 |
| BMI (kg/m2) | 25.2 ± 6.0 | 24.8 ± 3.9 | 25.5 ± 4.2 | <0.001 |
| SBP (mmHg) | 136.0 ± 27.9 | 130.3 ± 25.0 | 134.4 ± 26.7 | <0.001 |
| DBP (mmHg) | 79.3 ± 17.1 | 79.3 ± 14.8 | 79.5 ± 15.4 | 0.752 |
| Disease history | ||||
| DM | 50,176 (47.9%) | 984 (44.9%) | 1949 (59.2%) | <0.001 |
| HTN | 42,116 (40.2%) | 846 (38.6%) | 1773 (53.8%) | <0.001 |
| HLP | 41,117 (39.2%) | 880 (40.2%) | 1767 (53.7%) | <0.001 |
| CKD | 34,246 (32.7%) | 438 (20.0%) | 862 (26.2%) | <0.001 |
| STK | 13,893 (13.3%) | 216 (9.9%) | 430 (13.1%) | <0.001 |
| CAD | 24,474 (23.3%) | 508 (23.2%) | 1059 (32.2%) | <0.001 |
| HF | 6693 (6.4%) | 119 (5.4%) | 256 (7.8%) | 0.001 |
| AF | 4983 (4.8%) | 70 (3.2%) | 125 (3.8%) | <0.001 |
| COPD | 13,555 (12.9%) | 239 (10.9%) | 595 (18.1%) | <0.001 |
| Laboratory test | ||||
| HbA1c (%) | 7.0 ± 1.8 | 6.3 ± 1.4 | 6.6 ± 1.6 | <0.001 |
| GLU (mg/dL) | 119.1 ± 49.3 | 115.5 ± 43.9 | 123.2 ± 49.1 | <0.001 |
| eGFR (mL/min) | 81.6 ± 36.2 | 89.2 ± 27.1 | 84.5 ± 30.3 | <0.001 |
| BUN (mg/dL) | 22.1 ± 19.6 | 16.5 ± 9.7 | 18.8 ± 13.7 | <0.001 |
| Na (mmol/L) | 137.8 ± 4.8 | 139.0 ± 3.8 | 138.5 ± 4.2 | <0.001 |
| K (mmol/L) | 4.0 ± 0.5 | 4.1 ± 0.4 | 4.1 ± 0.5 | <0.001 |
| Cl (mEq/L) | 103.3 ± 5.0 | 103.8 ± 3.7 | 103.5 ± 4.4 | <0.001 |
| Ca (mg/dL) | 9.0 ± 0.7 | 9.2 ± 0.5 | 9.1 ± 0.6 | <0.001 |
| Mg (meq/L) | 2.1 ± 0.3 | 2.1 ± 0.2 | 2.1 ± 0.3 | 0.122 |
| Alb (g/dL) | 3.9 ± 0.7 | 4.2 ± 0.5 | 4.1 ± 0.5 | <0.001 |
| CRP (mg/L) | 2.8 ± 5.5 | 1.4 ± 3.3 | 1.8 ± 3.9 | <0.001 |
| WBC (103/uL) | 8.3 ± 5.1 | 7.0 ± 4.7 | 7.4 ± 3.2 | <0.001 |
| PLT (103/uL) | 235.4 ± 81.3 | 237.3 ± 68.1 | 234.9 ± 71.7 | 0.504 |
| Hb (mg/dL) | 13.1 ± 2.3 | 13.6 ± 1.9 | 13.5 ± 2.1 | <0.001 |
| AST (U/L) | 35.9 ± 119.8 | 22.3 ± 15.8 | 25.0 ± 21.8 | <0.001 |
| ALT (U/L) | 31.8 ± 103.2 | 22.5 ± 17.0 | 25.0 ± 25.6 | <0.001 |
| TG (mg/dL) | 136.6 ± 131.0 | 137.5 ± 104.7 | 145.7 ± 157.9 | <0.001 |
| TC (mg/dL) | 172.0 ± 48.8 | 179.4 ± 38.3 | 178.5 ± 41.7 | <0.001 |
| LDL (mg/dL) | 102.9 ± 37.5 | 108.2 ± 33.4 | 107.4 ± 34.9 | <0.001 |
| HDL (mg/dL) | 46.7 ± 15.2 | 49.4 ± 13.6 | 48.5 ± 14.0 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-S.; Lee, Y.-T.; Fang, W.-H.; Lou, Y.-S.; Kuo, F.-C.; Lee, C.-C.; Lin, C. Deep Learning Algorithm for Management of Diabetes Mellitus via Electrocardiogram-Based Glycated Hemoglobin (ECG-HbA1c): A Retrospective Cohort Study. J. Pers. Med. 2021, 11, 725. https://doi.org/10.3390/jpm11080725
Lin C-S, Lee Y-T, Fang W-H, Lou Y-S, Kuo F-C, Lee C-C, Lin C. Deep Learning Algorithm for Management of Diabetes Mellitus via Electrocardiogram-Based Glycated Hemoglobin (ECG-HbA1c): A Retrospective Cohort Study. Journal of Personalized Medicine. 2021; 11(8):725. https://doi.org/10.3390/jpm11080725
Chicago/Turabian StyleLin, Chin-Sheng, Yung-Tsai Lee, Wen-Hui Fang, Yu-Sheng Lou, Feng-Chih Kuo, Chia-Cheng Lee, and Chin Lin. 2021. "Deep Learning Algorithm for Management of Diabetes Mellitus via Electrocardiogram-Based Glycated Hemoglobin (ECG-HbA1c): A Retrospective Cohort Study" Journal of Personalized Medicine 11, no. 8: 725. https://doi.org/10.3390/jpm11080725
APA StyleLin, C.-S., Lee, Y.-T., Fang, W.-H., Lou, Y.-S., Kuo, F.-C., Lee, C.-C., & Lin, C. (2021). Deep Learning Algorithm for Management of Diabetes Mellitus via Electrocardiogram-Based Glycated Hemoglobin (ECG-HbA1c): A Retrospective Cohort Study. Journal of Personalized Medicine, 11(8), 725. https://doi.org/10.3390/jpm11080725