Perceptions about Research Participation among Individuals at Risk and Individuals with Premanifest Huntington’s Disease: A Survey Conducted by the European Huntington Association
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Survey
2.3. Statistical Analysis
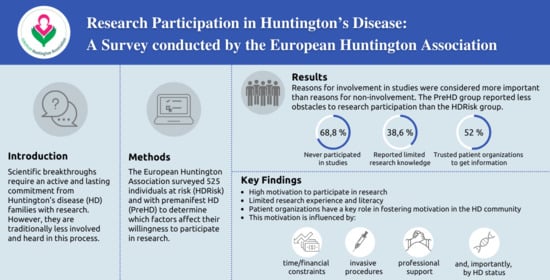
3. Results
3.1. Demographics
3.2. Level of Knowledge and Sources of Information about HD Research
3.3. Reasons for Involvement and Noninvolvement in Research
3.4. Factors Influencing HD Research Participation
4. Discussion
4.1. HD Research Experience and Literacy
4.2. Motivators for HD Research Participation
4.2.1. Reasons for Involvement in Research
4.2.2. Reasons for Noninvolvement in Research
4.2.3. Group-Specific Motivators of Research Participation
4.3. Moderators of HD Research Participation
4.3.1. Factors Facilitating Research Participation
4.3.2. Factors Preventing Research Participation
4.3.3. Group-Specific Moderators of Research Participation
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dash, D.; Mestre, T.A. Therapeutic Update on Huntington’s Disease: Symptomatic Treatments and Emerging Disease-Modifying Therapies. Neurotherapeutics 2020, 17, 1645–1659. [Google Scholar] [CrossRef]
- Bashir, H. Emerging therapies in Huntington’s disease. Expert Rev. Neurother. 2019, 19, 983–995. [Google Scholar] [CrossRef]
- Schoulson, I.; Young, A.B. Milestones in huntington disease. Mov. Disord. 2011, 26, 1127–1133. [Google Scholar] [CrossRef]
- European Huntington Association. Available online: https://eurohuntington.org/stronger-together-2/ (accessed on 2 March 2021).
- HD Trial Finder. Available online: https://hdtrialfinder.net/ (accessed on 2 March 2021).
- European Federation of Neurological Associations. Available online: https://www.efna.net/global-huntingtons-disease-patient-advocacy-organizations-unite-form-huntingtons-disease-coalition-patient-engagement-hd-cope/ (accessed on 2 March 2021).
- European Huntington Association. Available online: https://eurohuntington.org/2018/04/24/huge-progress-for-patient-representation/ (accessed on 2 March 2021).
- Landwehrmeyer, G.B.; Fitzer-Attas, C.J.; Giuliano, J.D.; Gonçalves, N.; Anderson, K.E.; Cardoso, F.; Ferreira, J.J.; Mestre, T.A.; Stout, J.C.; Sampaio, C. Data Analytics from Enroll-HD, a Global Clinical Research Platform for Huntington’s Disease. Mov. Disord. Clin. Pract. 2016, 4, 212–224. [Google Scholar] [CrossRef] [Green Version]
- Enroll-HD. Available online: https://enroll-hd.org/ (accessed on 4 March 2021).
- Tabrizi, S.J.; Leavitt, B.R.; Landwehrmeyer, G.B.; Wild, E.J.; Saft, C.; Barker, R.A.; Blair, N.F.; Craufurd, D.; Priller, J.; Rickards, H.; et al. Targeting Huntingtin Expression in Patients with Huntington’s Disease. N. Engl. J. Med. 2019, 380, 2307–2316. [Google Scholar] [CrossRef]
- Huntington’s Disease News. Available online: https://huntingtonsdiseasenews.com/2020/04/28/enrollment-complete-for-generation-hd1-trial-testing-tominersen-for-huntingtons/ (accessed on 15 March 2021).
- Wave Life Sciences. Available online: https://wavelifesciences.com/pipeline (accessed on 15 March 2021).
- GlobeNewswire. Available online: https://www.globenewswire.com/news-release/2020/07/07/2058502/0/en/Vaccinex-Provides-Update-of-Potentially-Pivotal-SIGNAL-Clinical-Trial-in-Huntington-s-Disease.html (accessed on 15 March 2021).
- Prilenia Therapeutics. Available online: https://www.prilenia.com/copy-of-18-september-2019-pr (accessed on 15 March 2021).
- UniQure. Available online: http://uniqure.com/patients/phase-1-2-clinical-trial-of-amt-130.php (accessed on 15 March 2021).
- American Parkinson Disease Association. Available online: https://www.apdaparkinson.org/article/parkinsons-clinical-trial-enrollment/ (accessed on 17 March 2021).
- Goodman, L.; Sia, C.; Carnes, R.; Vetter, L.; Taubman, F.; Venuto, C.; McGarry, A.; Kieburtz, K.; Agarwal, P. Advocacy Recruiting for Huntington’s Disease Clinical Trials. PLoS Curr. 2011, 12. [Google Scholar] [CrossRef]
- Food and Drug Administration. Available online: https://www.fdanews.com/ext/resources/files/2018/08-22-18-FDAsummary.pdf?1534970149%20 (accessed on 17 March 2021).
- Langbehn, D.R.; Hersch, S. Clinical Outcomes and Selection Criteria for Prodromal Huntington’s Disease Trials. Mov. Disord. 2020, 35, 2193–2200. [Google Scholar] [CrossRef]
- Baig, S.S.; Strong, M.; Rosser, E.; Taverner, N.V.; Glew, R.; Miedzybrodzka, Z.; Clarke, A.; Craufurd, D.; Quarrell, O.W.; UK Huntington’s Disease Prediction Consortium. 22 Years of predictive testing for Huntington’s disease: The experience of the UK Huntington’s Prediction Consortium. Eur. J. Hum. Genet. 2016, 24, 1396–1402. [Google Scholar] [CrossRef]
- Bernhardt, C.; Schwan, A.M.; Kraus, P.; Epplen, J.T.; Kunstmann, E. Decreasing uptake of predictive testing for Huntington’s disease in a German centre: 12 years’ experience (1993–2004). Eur. J. Hum. Genet. 2009, 17, 295–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampaio, C.; Levey, J.; Klitzman, R. Predictive testing and clinical trials in Huntington’s disease: An ethical analysis. Mov. Disord. 2018, 33, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Glidden, A.M.; Luebbe, E.A.; Elson, M.J.; Goldenthal, S.B.; Snyder, C.W.; Zizzi, C.E.; Dorsey, E.R.; Heatwole, C.R. Patient-reported impact of symptoms in Huntington disease: PRISM-HD. Neurology 2020, 94, e2045–e2053. [Google Scholar] [CrossRef]
- McCusker, E.A.; Gunn, D.G.; Epping, E.A.; Loy, C.T.; Radford, K.; Griffith, J.; Mills, J.A.; Long, J.D.; Paulsen, J.S.; PREDICT-HD Investigators of the Huntington Study Group. Unawareness of motor phenoconversion in huntington disease. Neurology 2013, 81, 1141–1147. [Google Scholar] [CrossRef] [Green Version]
- Nance, M.A. Genetic counseling and testing for Huntington’s disease: A historical review. Am. J. Med. Genet. 2017, 174, 75–92. [Google Scholar] [CrossRef]
- MacLeod, R.; Tibben, A.; Frontali, M.; Evers-Kiebooms, G.; Jones, A.; Martinez-Descales, A.; Roos, R.A.; Editorial Committee and Working Group ‘Genetic Testing Counselling’ of the European Huntington Disease Network. Recommendations for the predictive genetic test in Huntington’s disease. Clin. Genet. 2013, 83, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.D.; Bull, J.; Johnston McKee, K.; Mahon, E.; Harper, B.; Roberts, J.N.; CTTI Recruitment Project Team. Clinical trials recruitment planning: A proposed framework from the Clinical Trials Transformation Initiative. Contemp. Clin. Trials 2018, 66, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Boada, M.; Santos-Santos, M.A.; Rodríguez-Gómez, O.; Alegret, M.; Cañabate, P.; Lafuente, A.; Abdelnour, C.; Buendía, M.; de Dios, M.J.; Morera, A.; et al. Patient Engagement: The Fundació ACE Framework for Improving Recruitment and Retention in Alzheimer’s Disease Research. J. Alzheimers Dis. 2018, 62, 1079–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Huntington Association. Available online: http://eurohuntington.org/2020/10/26/moving-forward-towards-a-future-with-effective-treatment-for-huntingtons-disease/ (accessed on 1 April 2021).
- Paulsen, J.S.; Hayden, M.; Stout, J.C.; Langbehn, D.R.; Aylward, E.; Ross, C.A.; Guttman, M.; Nance, M.; Kieburtz, K.; Oakes, D.; et al. Preparing for preventive clinical trials: The Predict-HD study. Arch. Neurol. 2006, 63, 883–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, F.O. Huntington’s Disease. Semin. Neurol. 2007, 27, 143–150. [Google Scholar] [CrossRef]
- Reilmann, R.; Leavitt, B.R.; Ross, C.A. Diagnostic criteria for Huntington’s disease based on natural history. Mov. Disord. 2014, 29, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Survey Monkey. Available online: https://www.surveymonkey.com/ (accessed on 5 April 2021).
- Cotter, K.; Siskind, C.E.; Sha, S.J.; Hanson-Kahn, A.K. Positive Attitudes and Therapeutic Misconception Around Hypothetical Clinical Trial Participation in the Huntington’s Dis-ease Community. J. Huntingt. Dis. 2019, 8, 421–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elberse, J.E.; Pittens, C.A.; de Cock Buning, T.; Broerse, J.E. Patient involvement in a scientific advisory process: Setting the research agenda for medical products. Health Policy 2012, 107, 231–242. [Google Scholar] [CrossRef]
- Goodman, L.; Guiliano, J.; Lovecky, D. Survey of Clinical Trial Interest and Literacy in Huntington Support Groups: Northwest Pilot Project. Neurotherapeutics 2009, 6, 204. [Google Scholar] [CrossRef]
- Reijula, E.; Pietilä, A.M.; Halkoaho, A.; Selander, T.; Martikainen, K.; Kälviäinen, R.; Keränen, T. Clinical features of Parkinson’s disease patients are associated with therapeutic misconception and willingness to participate in clinical trials. Trials 2017, 18, 444. [Google Scholar] [CrossRef]
- de Melo-Martín, I.; Holtzman, M.; Hacker, K.S. “I Want to Do It, But I Want to Make Sure That I Do It Right.” Views of Patients with Parkinson’s Disease Regarding Early Stem Cell Clinical Trial Participation. AJOB Empir. Bioeth. 2020, 11, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Hoos, A.; Anderson, J.; Boutin, M.; Dewulf, L.; Geissler, J.; Johnston, G.; Joos, A.; Metcalf, M.; Regnante, J.; Sargeant, I.; et al. Partnering With Patients in the Development and Lifecycle of Medicines: A Call for Action. Ther. Innov. Regul. Sci. 2015, 49, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Grace Cannard, K.; Hacker, M.L.; Molinari, A.; Heusinkveld, L.E.; Currie, A.D.; Charles, D. Recruitment and Retention in Clinical Trials of Deep Brain Stimulation in Early-Stage Parkinson’s Disease: Past Experiences and Future Considerations. J. Parkinsons Dis. 2018, 8, 421–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bardakjian, T.M.; Naczi, K.F.; Gonzalez-Alegre, P. Attitudes of Potential Participants Towards Molecular Therapy Trials in Huntington’s Disease. J. Huntingt. Dis. 2019, 8, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Cleret de Langavant, L.; Sudraud, S.; Verny, C.; Krystkowiak, P.; Simonin, C.; Damier, P.; Demonet, J.F.; Supiot, F.; Rialland, A.; Schmitz, D.; et al. Longitudinal study of informed consent in innovative therapy research: Experience and provisional recommendations from a multicenter trial of intracerebral grafting. PLoS ONE 2015, 10, e0128209. [Google Scholar] [CrossRef]
- Burns, K.E.A.; Magyarody, N.; Jiang, D.; Wald, R. Attitudes and views of the general public towards research participation. Intern. Med. J. 2013, 43, 531–540. [Google Scholar] [CrossRef]
- Appelbaum, P.; Anatchkova, M.; Albert, K.; Dunn, L.; Lidz, C. Therapeutic misconception in research subjects: Development and validation of a measure. Clin. Trials 2012, 9, 748–761. [Google Scholar] [CrossRef] [Green Version]
- Horng, S.; Grady, C. Misunderstanding in clinical research: Distinguishing therapeutic misconception, therapeutic misestimation, & therapeutic optimism. IRB 2003, 25, 11–16. [Google Scholar] [PubMed]
- Eccles, F.; Craufurd, D.; Smith, A.; Davies, R.; Glenny, K.; Homberger, M.; Rose, L.; Theed, R.; Peeren, S.; Rogers, D.; et al. Experiences of Mindfulness-Based Cognitive Therapy for Premanifest Huntington’s Disease. J. Huntingt. Dis. 2021, 10, 277–291. [Google Scholar] [CrossRef]
- Oliveri, S.; Ferrari, F.; Manfrinati, A.; Pravettoni, G. A Systematic Review of the Psychological Implications of Genetic Testing: A Comparative Analysis Among Cardiovascular, Neurodegenerative and Cancer Diseases. Front. Genet. 2018, 9, 624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wusthoff, C. The dilemma of confidentiality in Huntington disease. JAMA 2003, 290, 1219–1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, K.E.; Eberly, S.; Marder, K.S.; Oakes, D.; Kayson, E.; Young, A.; Shoulson, I.; PHAROS Investigators. The choice not to undergo genetic testing for Huntington disease: Results from the PHAROS study. Clin. Genet. 2019, 96, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Davies, E.; Craufurd, D.; MacLeod, R. “It’s being part of the big picture, even though you’re a tiny jigsaw piece”-motivations and expectations of individuals participating in the Enroll-HD observational study. J. Community Genet. 2020, 11, 421–432. [Google Scholar] [CrossRef] [Green Version]
- Grill, J.D.; Karlawish, J. Addressing the challenges to successful recruitment and retention in Alzheimer’s disease clinical trials. Alzheimers Res. Ther. 2010, 2, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kjoelaas, S.; Tillerås, K.H.; Feragen, K.B. The Ripple Effect: A Qualitative Overview of Challenges When Growing Up in Families Affected by Huntington’s Disease. J. Huntingt. Dis. 2020, 9, 129–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forrest Keenan, K.; Miedzybrodzka, Z.; van Teijlingen, E.; McKee, L.; Simpson, S.A. Young people’s experiences of growing up in a family affected by Huntington’s disease. Clin. Genet. 2007, 71, 120–129. [Google Scholar] [CrossRef]
- Klitzman, R.; Thorne, D.; Williamson, J.; Chung, W.; Marder, K. Decision-making about reproductive choices among individuals at-risk for Huntington’s disease. J. Genet. Couns. 2007, 16, 347–362. [Google Scholar] [CrossRef]
- Ho, A.; Robbins, A.O.G.; Barker, R.A. Huntington’s disease patients have selective problems with insight. Mov. Disord. 2006, 21, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Sitek, E.J.; Sołtan, W.; Wieczorek, D.; Schinwelski, M.; Robowski, P.; Harciarek, M.; Guzińska, K.; Sławek, J. Self-awareness of executive dysfunction in Huntington’s disease: Comparison with Parkinson’s disease and cervical dystonia. Psychiatry Clin. Neurosci. 2013, 67, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Snowden, J.S.; Craufurd, D.; Griffiths, H.L.; Neary, D. Awareness of involuntary movements in Huntington disease. Arch. Neurol. 1998, 55, 801–805. [Google Scholar] [CrossRef] [Green Version]
- Tedroff, J.; Waters, S.; Barker, R.A.; Roos, R.; Squitieri, F.; EHDN Registry Study Group. Antidopaminergic Medication is Associated with More Rapidly Progressive Huntington’s Disease. J. Huntingt. Dis. 2015, 4, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Vaccarino, A.L.; Sills, T.; Anderson, K.E.; Biglan, K.; Borowsky, B.; Giuliano, J.; Guttman, M.; Ho, A.K.; Kennard, C.; Kupchak, P.; et al. Assessment of motor symptoms and functional impact in prodromal and early huntington disease. PLoS Curr. 2011, 2, RRN1244. [Google Scholar] [CrossRef] [PubMed]

| Demographics | ||||
|---|---|---|---|---|
| Total n = 525 | HDRisk n = 263 | PreHD n = 262 | Chi-Square HDRisk vs. PreHD | |
| % | % | % | χ2 (p-value) | |
| Gender | ||||
| Female | 71.6 | 73.4 | 69.8 | 0.808 (0.369) |
| Male | 28.4 | 26.6 | 30.2 | |
| Other | 0 | 0 | 0 | - |
| Age Interval (years) | ||||
| 18 to 24 | 8.6 | 12.5 | 4.6 | 10.631 (0.001) ** |
| 25 to 34 | 29.5 | 27.8 | 31.3 | 0.791 (0.374) |
| 35 to 44 | 33.1 | 35 | 31.3 | 0.804 (0.370) |
| 45 to 54 | 18.3 | 18.3 | 18.3 | 0.000 (0.984) |
| 55 to 64 | 8.4 | 5.7 | 11.1 | 4.920 (0.027) * |
| 65 to 74 | 1.5 | 0.8 | 2.3 | 2.046 (0.153) |
| 75 or older | 0.6 | 0 | 1.1 | 3.029 (0.082) |
| Education Level | ||||
| Did not attend school | 0 | 0 | 0 | - |
| Completed primary education | 1 | 0.4 | 1.5 | 1.829 (0.176) |
| Completed secondary education | 20.4 | 18.3 | 22.5 | 1.473 (0.225) |
| Graduated from high school | 28.4 | 27.8 | 29 | 0.101 (0.751) |
| Graduated from college | 26.5 | 29.7 | 23.3 | 2.740 (0.098) |
| Completed graduate school | 23.8 | 24 | 23.7 | 0.006 (0.938) |
| Previous HD Research Experience | ||||
| Yes | 31.2 | 16.3 | 46.2 | 54.384 (<0.001) ** |
| No | 68.8 | 83.7 | 53.8 | |
| Knowledge and Information about HD Research | ||||
|---|---|---|---|---|
| Total n = 525 | HDRisk n = 263 | PreHD n = 262 | Chi-Square HDRisk vs. PreHD | |
| % | % | % | χ2 (p-value) | |
| Knowledge about HD Research | ||||
| Not good | 5.3 | 6.5 | 4.2 | 1.334 (0.248) |
| Should be better | 33.3 | 39.2 | 27.5 | 8.061 (0.005) ** |
| Satisfactory | 24.6 | 26.2 | 22.9 | 0.788 (0.375) |
| Good | 28.6 | 23.2 | 34 | 7.467 (0.006) ** |
| Excellent | 7.8 | 4.9 | 10.7 | 6.015 (0.014) * |
| Do not want to know about HD research | 0.4 | 0 | 0.8 | 2.015 (0.156) |
| Sources of Information about HD Research | ||||
| Internet | 78.5 | 78.7 | 78.2 | 0.017 (0.897) |
| Television | 4 | 2.7 | 5.3 | 2.458 (0.117) |
| Press/newsletters/flyers/booklets | 19.8 | 22.4 | 17.2 | 2.284 (0.131) |
| HD associations and/or support groups | 52 | 51 | 53.1 | 0.233 (0.630) |
| Healthcare professionals | 25.5 | 23.2 | 27.9 | 1.505 (0.220) |
| Family members | 25.3 | 30.8 | 19.8 | 8.321 (0.004) ** |
| Not interested in HD research information | 0.4 | 0.4 | 0.4 | 0.000 (0.998) |
| Factors Influencing Research Participation | ||||
|---|---|---|---|---|
| Total n = 525 | HDRisk n = 263 | PreHD n = 262 | Chi-Square HDRisk vs. PreHD | |
| % | % | % | χ2 (p-value) | |
| Factors Preventing Participation | ||||
| I would have to travel to the study site | 29.9 | 30.8 | 29 | 0.201 (0.654) |
| I would not have family support | 5.5 | 5.7 | 5.3 | 0.033 (0.857) |
| I would have personal expenses | 33.1 | 34.6 | 31.7 | 0.506 (0.477) |
| I would have to do motor exams | 3.2 | 4.6 | 1.9 | 2.951 (0.086) |
| I would have to find someone to take care of my sick parent | 7 | 11 | 3.1 | 12.737 (<0.001) ** |
| I would have to skip workdays/hours | 30.3 | 36.1 | 24.4 | 8.501 (0.004) ** |
| I would have to be involved in the study for a long time | 14.1 | 19 | 9.2 | 10.519 (0.001) ** |
| I would have to go through invasive procedures | 31.4 | 36.9 | 26 | 7.273 (0.007) ** |
| I would have to do cognitive assessments | 4 | 5.7 | 2.3 | 3.982 (0.046) * |
| I would have to find someone to take care of my children | 11.8 | 11.4 | 12.2 | 0.082 (0.775) |
| I would have to rethink my decisions about family planning | 11.2 | 17.5 | 5 | 20.653 (<0.001) ** |
| None of the above | 28.6 | 22.8 | 34.4 | 8.561 (0.003) ** |
| Factors Facilitating Participation | ||||
| I would have assistance from a patient advocate | 25.7 | 26.2 | 25.2 | 0.075 (0.784) |
| I would have family support | 26.9 | 25.9 | 27.9 | 0.269 (0.604) |
| The researcher is my doctor/my family member doctor | 17.1 | 17.5 | 16.8 | 0.045 (0.832) |
| My personal expenses would be reimbursed | 42.5 | 41.1 | 43.9 | 0.430 (0.512) |
| I would get the chance to meet other HD families | 27 | 27 | 27.1 | 0.001 (0.979) |
| I would gain easier access to treatments and health professionals | 61.7 | 62.7 | 60.7 | 0.234 (0.629) |
| I would have psychological and social care available | 50.7 | 55.5 | 45.8 | 4.953 (0.026) * |
| I would not have to go through invasive procedures | 26.5 | 31.6 | 21.4 | 6.994 (0.008) ** |
| I know and trust the study team | 36.8 | 35.7 | 37.8 | 0.236 (0.627) |
| I would have regular feedback about my health condition | 55.4 | 53.6 | 57.3 | 0.704 (0.402) |
| None of the above | 7 | 6.5 | 7.6 | 0.274 (0.601) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Júlio, F.; Blanco, R.; Casanova, J.P.; D’Alessio, B.; De Schepper, B.; De Sousa, D.; De Sousa, P.; Ferreira, C.; Gommans, H.; Haselberg, R.; et al. Perceptions about Research Participation among Individuals at Risk and Individuals with Premanifest Huntington’s Disease: A Survey Conducted by the European Huntington Association. J. Pers. Med. 2021, 11, 815. https://doi.org/10.3390/jpm11080815
Júlio F, Blanco R, Casanova JP, D’Alessio B, De Schepper B, De Sousa D, De Sousa P, Ferreira C, Gommans H, Haselberg R, et al. Perceptions about Research Participation among Individuals at Risk and Individuals with Premanifest Huntington’s Disease: A Survey Conducted by the European Huntington Association. Journal of Personalized Medicine. 2021; 11(8):815. https://doi.org/10.3390/jpm11080815
Chicago/Turabian StyleJúlio, Filipa, Ruth Blanco, Josè Perez Casanova, Barbara D’Alessio, Beatrice De Schepper, Dina De Sousa, Paul De Sousa, Cristina Ferreira, Hans Gommans, Rob Haselberg, and et al. 2021. "Perceptions about Research Participation among Individuals at Risk and Individuals with Premanifest Huntington’s Disease: A Survey Conducted by the European Huntington Association" Journal of Personalized Medicine 11, no. 8: 815. https://doi.org/10.3390/jpm11080815
APA StyleJúlio, F., Blanco, R., Casanova, J. P., D’Alessio, B., De Schepper, B., De Sousa, D., De Sousa, P., Ferreira, C., Gommans, H., Haselberg, R., Hermant, E., Lis, D., Maffi, S., Olsen, S. O., Papantoniou, M., Squitieri, F., Tretyakova, M., Umakhanova, Z., Václavík, V., ... on behalf of the European Huntington Association. (2021). Perceptions about Research Participation among Individuals at Risk and Individuals with Premanifest Huntington’s Disease: A Survey Conducted by the European Huntington Association. Journal of Personalized Medicine, 11(8), 815. https://doi.org/10.3390/jpm11080815