Personalized Target Heart Rate for Patients with Heart Failure and Reduced Ejection Fraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Endpoint
2.3. Statistical Analysis
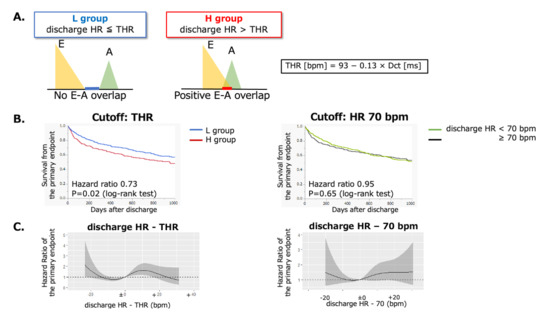
3. Results
3.1. Long-Term Outcomes in the L and H Groups
3.2. Restricted Cubic Spline Curves for the Function Relating Discharge HR or THR and the Endpoints
3.3. Stratified Analysis
4. Discussion
4.1. Association of HR with E-A Overlap and Hemodynamics
4.2. Clinical Significance of THR in Patients with HFrEF
4.3. Association of Achieving THR and BMI in Patients with HFrEF
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shiraishi, Y.; Kohsaka, S.; Sato, N.; Takano, T.; Kitai, T.; Yoshikawa, T.; Matsue, Y. 9-Year Trend in the Management of Acute Heart Failure in Japan: A Report from the National Consortium of Acute Heart Failure Registries. J. Am. Heart Assoc. 2018, 7, e008687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takada, T.; Sakata, Y.; Miyata, S.; Takahashi, J.; Nochioka, K.; Miura, M.; Tadaki, S.; Shimokawa, H. Impact of elevated heart rate on clinical outcomes in patients with heart failure with reduced and preserved ejection fraction: A report from the CHART-2 Study. Eur. J. Heart Fail. 2014, 16, 309–316. [Google Scholar] [CrossRef] [PubMed]
- McAlister, F.A.; Wiebe, N.; Ezekowitz, J.A.; Leung, A.A.; Armstrong, P.W. Meta-analysis: Beta-blocker dose, heart rate reduction, and death in patients with heart failure. Ann. Intern. Med. 2009, 150, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, D.; Flather, M.D.; Altman, D.G.; Holmes, J.; Rosano, G.; Wikstrand, J.; Packer, M.; Coats, A.J.S.; Manzano, L.; Böhm, M.; et al. Heart Rate and Rhythm and the Benefit of Beta-Blockers in Patients with Heart Failure. J. Am. Coll. Cardiol. 2017, 69, 2885–2896. [Google Scholar] [CrossRef] [PubMed]
- Nagatomo, Y.; Yoshikawa, T.; Okamoto, H.; Kitabatake, A.; Hori, M. Differential Response to Heart Rate Reduction by Carvedilol in Heart Failure and Reduced Ejection Fraction between Sinus Rhythm and Atrial Fibrillation—Insight from J-CHF Study. Circ. Rep. 2020, 2, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Swedberg, K.; Komajda, M.; Böhm, M.; Borer, J.S.; Ford, I.; Dubost-Brama, A.; Lerebours, G.; Tavazzi, L. Ivabradine and outcomes in chronic heart failure (SHIFT): A randomised placebo-controlled study. Lancet 2010, 376, 875–885. [Google Scholar] [CrossRef]
- Böhm, M.; Swedberg, K.; Komajda, M.; Borer, J.S.; Ford, I.; Dubost-Brama, A.; Lerebours, G.; Tavazzi, L. Heart rate as a risk factor in chronic heart failure (SHIFT): The association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet 2010, 376, 886–894. [Google Scholar] [CrossRef]
- Chung, C.S.; Kovács, S.J. Consequences of increasing heart rate on deceleration time, the velocity-time integral, and E/A. Am. J. Cardiol. 2006, 97, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Appleton, C.P. Influence of incremental changes in heart rate on mitral flow velocity: Assessment in lightly sedated, conscious dogs. J. Am. Coll. Cardiol. 1991, 17, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Izumida, T.; Imamura, T.; Nakamura, M.; Fukuda, N.; Kinugawa, K. How to consider target heart rate in patients with systolic heart failure. ESC Heart Fail. 2020, 7, 3231–3234. [Google Scholar] [CrossRef] [PubMed]
- McKee, P.A.; Castelli, W.P.; McNamara, P.M.; Kannel, W.B. The natural history of congestive heart failure: The Framingham study. N. Engl. J. Med. 1971, 285, 1441–1446. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Ghaleh, B.; Puybasset, L.; Giudicelli, J.F.; Berdeaux, A. Coronary and hemodynamic effects of S 16257, a new bradycardic agent, in resting and exercising conscious dogs. J. Pharmacol. Exp. Ther. 1995, 275, 659–666. [Google Scholar] [PubMed]
- Sato, M.; Hoka, S.; Arimura, H.; Ono, K.; Yoshitake, J. Effects of augmenting cardiac contractility, preload, and heart rate on cardiac output during enflurane anesthesia. Anesth. Analg. 1991, 73, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.S.; Karamanoglu, M.; Kovács, S.J. Duration of diastole and its phases as a function of heart rate during supine bicycle exercise. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H2003–H2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusunose, K.; Arase, M.; Zheng, R.; Hirata, Y.; Nishio, S.; Ise, T.; Yamaguchi, K.; Fukuda, D.; Yagi, S.; Yamada, H.; et al. Clinical Utility of Overlap Time for Incomplete Relaxation to Predict Cardiac Events in Heart Failure: Incomplete relaxation in heart failure. J. Card. Fail. 2021, 27, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Izumida, T.; Imamura, T.; Ueno, Y.; Tanaka, S.; Kataoka, N.; Nakamura, M.; Kinugawa, K. Impact of optimal heart rate on left ventricular reverse remodeling and functional improvement in patients with systolic heart failure. Heart Vessel. 2021, 36, 1688–1693. [Google Scholar] [CrossRef] [PubMed]
- St Goar, F.G.; Masuyama, T.; Alderman, E.L.; Popp, R.L. Left ventricular diastolic dysfunction in end-stage dilated cardiomyopathy: Simultaneous Doppler echocardiography and hemodynamic evaluation. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 1991, 4, 349–360. [Google Scholar] [CrossRef]
- DeSanctis, R.W. Diagnostic and therapeutic uses of atrial pacing. Circulation 1971, 43, 748–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, N.; Ishikawa, T.; Sumita, S.; Nakagawa, T.; Kobayashi, T.; Matsushita, K.; Matsumoto, K.; Ohkusu, Y.; Taima, M.; Kosuge, M.; et al. Long-term follow-up of atrioventricular delay optimization in patients with biventricular pacing. Circ. J. 2005, 69, 201–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Variable | Overall (N = 647) | L Group (N = 328) | H Group (N = 319) | p-Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 72 (59, 80) | 71 (58, 80) | 72 (60, 81) | 0.21 |
| Sex (Male) | 452 (70%) | 242 (74%) | 210 (66%) | 0.028 |
| BMI, kg/m2 | 22.9 (20.3, 25.7) | 23.2 (20.8, 25.9) | 22.1 (19.9, 25.1) | 0.017 |
| Etiology DCM/ICM/VHD | 191/272/72 (30%/42%/11%) | 99/142/26 (30%/43%/8%) | 92/130/46 (29%/41%/14%) | 0.066 |
| Medical history | ||||
| History of ADHF hospitalization | 200 (31%) | 97 (30%) | 103 (32%) | 0.47 |
| HT | 422 (65%) | 209 (64%) | 213 (67%) | 0.42 |
| DLp | 289 (45%) | 142 (44%) | 147 (47%) | 0.49 |
| DM | 266 (41%) | 134 (41%) | 132 (41%) | 0.89 |
| Smoking | 302 (48%) | 155 (49%) | 147 (47%) | 0.65 |
| HD | 21 (3%) | 8 (2%) | 13 (4%) | 0.24 |
| COPD | 27 (4%) | 11 (3%) | 16 (5%) | 0.30 |
| HOT | 23 (4%) | 13 (4%) | 10 (3%) | 0.57 |
| Stroke/TIA | 78 (12%) | 28 (9%) | 50 (16%) | 0.005 |
| Pacemaker | 33 (5%) | 13 (4%) | 20 (6%) | 0.18 |
| ICD | 37 (6%) | 26 (8%) | 11 (3%) | 0.013 |
| CRT | 18 (3%) | 12 (4%) | 6 (2%) | 0.17 |
| Clinical profiles at admission | ||||
| NYHA (II–III/IV [%]) | 342/297 (54%/46%) | 168/155 (52%/48%) | 174/142 (55%/45%) | 0.44 |
| SBP | 136 (114, 160) | 134 (112, 160) | 140 (116, 159) | 0.71 |
| DBP | 81 (68, 99) | 81 (66, 98) | 82 (70, 100) | 0.77 |
| HR | 95 (78, 110) | 90 (72, 108) | 99 (80, 110) | 0.006 |
| Labs at admission | ||||
| BNP | 937 (482, 1592) | 951 (468, 1628) | 913 (494, 1592) | 0.94 |
| NT-proBNP | 5727 (3332, 12,357) | 5544 (3065, 11,379) | 6580 (3732, 14,561) | 0.29 |
| Alb | 3.7 (3.3, 4.0) | 3.7 (3.4, 4.1) | 3.6 (3.3, 3.9) | 0.026 |
| Hb | 12.6 (11.1, 14.4) | 12.9 (11.2, 14.6) | 12.4 (10.9, 14.0) | 0.030 |
| BUN | 21.4 (16.3, 30.7) | 22.2 (16.6, 31.2) | 20.9 (16.1, 30.0) | 0.91 |
| Cr | 1.1 (0.8, 1.4) | 1.1 (0.9, 1.4) | 1.0 (0.8, 1.5) | 0.41 |
| eGFR | 51.2 (35.1, 65.1) | 50.8 (36.7, 64.1) | 52.7 (32.1, 66.8) | 0.76 |
| UA | 6.8 (5.6, 8.4) | 7.1 (5.7, 8.5) | 6.7 (5.5, 8.0) | 0.09 |
| Na | 140 (137, 142) | 140 (137, 142) | 140 (137, 142) | 0.84 |
| TB | 0.8 (0.6, 1.3) | 0.9 (0.6, 1.4) | 0.8 (0.6, 1.1) | 0.007 |
| Echocardiography | ||||
| LVDd | 59 (53, 65) | 59 (55, 65) | 59 (52, 65) | 0.29 |
| LVDs | 51 (45, 57) | 51 (46, 58) | 51 (44, 57) | 0.48 |
| LVEF | 30 (23, 35) | 29 (23, 35) | 30 (23, 35) | 0.28 |
| LAD | 42 (37, 47) | 44 (38, 48) | 42 (37, 46) | 0.004 |
| E/e’ | 17.2 (10.8–24.7) | 18.0 (11.0–26.0) | 16.7 (10.5–23.6) | 0.12 |
| TRPG | 28 (21–36) | 29 (22–37) | 27 (19–35) | 0.014 |
| Dct | 152 (121, 190) | 140 (114, 170) | 176 (137, 218) | <0.001 |
| THR | 73 (68, 77) | 75 (71, 78) | 70 (65, 75) | <0.001 |
| Variable | Overall (N = 647) | L Group (N = 328) | H Group (N = 319) | p Value |
|---|---|---|---|---|
| Clinical profiles at discharge | ||||
| SBP | 108 (96, 120) | 106 (94, 119) | 107 (96, 120) | 0.18 |
| HR | 72 (64, 80) | 66 (60, 71) | 80 (74, 86) | <0.001 |
| Length of hospital stay | 16 (11–25) | 16 (11–25) | 17 (11–26) | 0.99 |
| Medication at discharge | ||||
| β-blocker | 566 (87.5%) | 295 (89.9%) | 271 (85.0%) | 0.055 |
| β-blocker dose (mg carvedilol) | 2.5 (1.25, 6.25) | 3.75 (1.25, 7.5) | 2.5 (1.25, 5) | 0.085 |
| β-blocker dose/kg BW | 0.057 (0.025, 0.117) | 0.059 (0.026, 0.122) | 0.055 (0.024, 0.107) | 0.19 |
| RAS inhibitor | 455 (70%) | 232 (71%) | 223 (70%) | 0.82 |
| MRA | 297 (46%) | 145 (44%) | 152 (48%) | 0.40 |
| Amiodarone | 82 (13%) | 52 (16%) | 30 (9%) | 0.013 |
| Digoxin | 9 (1.4%) | 7 (2%) | 2 (0.6%) | 0.09 |
| Loop diuretics | 500 (77%) | 258 (79%) | 242 (76%) | 0.36 |
| Loop diuretics (dose, mg) | 20 (20–40) | 20 (20–40) | 20 (20–40) | 0.69 |
| Tolvaptan | 34 (6.0%) | 13 (4.4%) | 22 (7.4%) | 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yumita, Y.; Nagatomo, Y.; Takei, M.; Saji, M.; Goda, A.; Kohno, T.; Nakano, S.; Nishihata, Y.; Ikegami, Y.; Shiraishi, Y.; et al. Personalized Target Heart Rate for Patients with Heart Failure and Reduced Ejection Fraction. J. Pers. Med. 2022, 12, 50. https://doi.org/10.3390/jpm12010050
Yumita Y, Nagatomo Y, Takei M, Saji M, Goda A, Kohno T, Nakano S, Nishihata Y, Ikegami Y, Shiraishi Y, et al. Personalized Target Heart Rate for Patients with Heart Failure and Reduced Ejection Fraction. Journal of Personalized Medicine. 2022; 12(1):50. https://doi.org/10.3390/jpm12010050
Chicago/Turabian StyleYumita, Yusuke, Yuji Nagatomo, Makoto Takei, Mike Saji, Ayumi Goda, Takashi Kohno, Shintaro Nakano, Yosuke Nishihata, Yukinori Ikegami, Yasuyuki Shiraishi, and et al. 2022. "Personalized Target Heart Rate for Patients with Heart Failure and Reduced Ejection Fraction" Journal of Personalized Medicine 12, no. 1: 50. https://doi.org/10.3390/jpm12010050
APA StyleYumita, Y., Nagatomo, Y., Takei, M., Saji, M., Goda, A., Kohno, T., Nakano, S., Nishihata, Y., Ikegami, Y., Shiraishi, Y., Kohsaka, S., Adachi, T., & Yoshikawa, T. (2022). Personalized Target Heart Rate for Patients with Heart Failure and Reduced Ejection Fraction. Journal of Personalized Medicine, 12(1), 50. https://doi.org/10.3390/jpm12010050