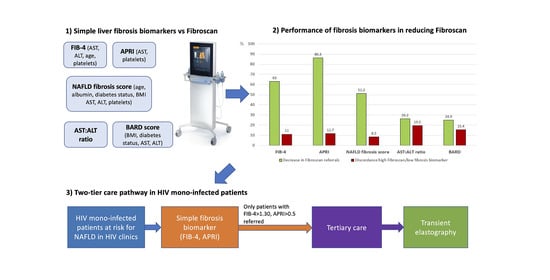
Two-Tier Care Pathways for Liver Fibrosis Associated to Non-Alcoholic Fatty Liver Disease in HIV Mono-Infected Patients
Abstract
:1. Introduction
2. Materials and Methods
- -
- -
- AST-to-Platelet Ratio Index (APRI) = (AST/upper limit of normal)/platelets × 100. A cut-off < 0.5 was used to exclude significant liver fibrosis [36].
- -
- -
- BARD = BMI ≥ 28 kg/m2 (yes = 1, no = 0) + AST:ALT ratio ≥ 0.8 (yes = 2, no = 0) + type-2 diabetes (yes = 1, no = 0). A cut-off < 2 was used to exclude significant liver fibrosis [38].
- -
- NAFLD fibrosis score = −1.675 + (0.037 × age) + (0.094 × BMI) + (1.13 × diabetes (yes = 1, no = 0)) + (0.99 × AST:ALT ratio) − (0.013 × platelets) − (0.66 × albumin (g/dL)). A cut off < −1.455 was used to exclude significant liver fibrosis [39].
3. Results
3.1. Application of the Two-Tier Pathway
3.2. Effect of NAFLD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rinella, M.; Charlton, M. The globalization of nonalcoholic fatty liver disease: Prevalence and impact on world health. Hepatology 2016, 64, 19–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, J.; Osikowicz, M.; Sebastiani, G. Clinical significance of elevated liver transaminases in HIV-infected patients. AIDS 2019, 33, 1267–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemoine, M.; Serfaty, L.; Capeau, J. From nonalcoholic fatty liver to nonalcoholic steatohepatitis and cirrhosis in HIV-infected patients: Diagnosis and management. Curr. Opin. Infect. Dis. 2012, 25, 10–16. [Google Scholar] [CrossRef]
- Maurice, J.B.; Patel, A.; Scott, A.J.; Patel, K.; Thursz, M.; Lemoine, M. Prevalence and risk factors of nonalcoholic fatty liver disease in HIV-monoinfection. AIDS 2017, 31, 1621–1632. [Google Scholar] [CrossRef] [PubMed]
- Sterling, R.K.; Smith, P.G.; Brunt, E.M. Hepatic steatosis in human immunodeficiency virus: A prospective study in patients without viral hepatitis, diabetes, or alcohol abuse. J. Clin. Gastroenterol. 2013, 47, 182–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crum-Cianflone, N.; Dilay, A.; Collins, G.; Asher, D.; Campin, R.; Medina, S.; Goodman, Z.; Parker, R.; Lifson, A.; Capozza, T.; et al. Nonalcoholic fatty liver disease among HIV-infected persons. J. Acquir. Immune Defic. Syndr. 2009, 50, 464–473. [Google Scholar] [CrossRef] [Green Version]
- Vuille-Lessard, E.; Lebouche, B.; Lennox, L.; Routy, J.P.; Costiniuk, C.T.; Pexos, C.; Giannakis, A.; Szabo, J.; Klein, M.B.; Sebastiani, G. Nonalcoholic fatty liver disease diagnosed by transient elastography with controlled attenuation parameter in unselected HIV monoinfected patients. AIDS 2016, 30, 2635–2643. [Google Scholar]
- Guaraldi, G.; Lonardo, A.; Maia, L.; Palella, F.J., Jr. Metabolic concerns in aging HIV-infected persons: From serum lipid phenotype to fatty liver. AIDS 2017, 31 (Suppl. 2), S147–S156. [Google Scholar] [CrossRef]
- Rockstroh, J.K.; Mohr, R.; Behrens, G.; Spengler, U. Liver fibrosis in HIV: Which role does HIV itself, long-term drug toxicities and metabolic changes play? Curr. Opin. HIV AIDS 2014, 9, 365–370. [Google Scholar] [CrossRef]
- Pembroke, T.; Deschenes, M.; Lebouche, B.; Benmassaoud, A.; Sewitch, M.; Ghali, P.; Wong, P.; Halme, A.; Vuille-Lessard, E.; Pexos, C.; et al. Hepatic steatosis progresses faster in HIV mono-infected than HIV/HCV co-infected patients and is associated with liver fibrosis. J. Hepatol. 2017, 67, 801–808. [Google Scholar] [CrossRef] [Green Version]
- Vodkin, I.; Valasek, M.A.; Bettencourt, R.; Cachay, E.; Loomba, R. Clinical, biochemical and histological differences between HIV-associated NAFLD and primary NAFLD: A case-control study. Aliment. Pharm. 2015, 41, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, M.; Lacombe, K.; Bastard, J.P.; Sebire, M.; Fonquernie, L.; Valin, N.; Fellahi, S.; Capeau, J.; Girard, P.M.; Meynard, J.L. Metabolic syndrome and obesity are the cornerstones of liver fibrosis in HIV-monoinfected patients: Results of the METAFIB study. AIDS 2017, 31, 1955–1964. [Google Scholar] [CrossRef] [PubMed]
- Benmassaoud, A.; Ghali, P.; Cox, J.; Wong, P.; Szabo, J.; Deschenes, M.; Osikowicz, M.; Lebouche, B.; Klein, M.B.; Sebastiani, G. Screening for nonalcoholic steatohepatitis by using cytokeratin 18 and transient elastography in HIV mono-infection. PLoS ONE 2018, 13, e0191985. [Google Scholar] [CrossRef]
- Price, J.C.; Seaberg, E.C.; Badri, S.; Witt, M.D.; D’Acunto, K.; Thio, C.L. HIV monoinfection is associated with increased aspartate aminotransferase-to-platelet ratio index, a surrogate marker for hepatic fibrosis. J. Infect. Dis. 2012, 205, 1005–1013. [Google Scholar] [CrossRef] [Green Version]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Taylor, R.S.; Taylor, R.J.; Bayliss, S.; Hagstrom, H.; Nasr, P.; Schattenberg, J.M.; Ishigami, M.; Toyoda, H.; Wong, V.W.-S.; Peleg, N.; et al. Association Between Fibrosis Stage and Outcomes of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterology 2020, 158, 1611–1625.e1612. [Google Scholar] [CrossRef] [Green Version]
- Ryom, L.; Cotter, A.; De Miguel, R.; Beguelin, C.; Podlekareva, D.; Arribas, J.R.; Marzolini, C.; Mallon, P.; Rauch, A.; Kirk, O.; et al. 2019 update of the European AIDS Clinical Society Guidelines for treatment of people living with HIV version 10.0. HIV Med. 2020, 21, 617–624. [Google Scholar] [CrossRef]
- Introduction: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42 (Suppl. 1), S1–S2. [CrossRef] [Green Version]
- European Association for the Study of The Liver; European Association for the Study of Diabetes. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Cervo, A.; Shengir, M.; Patel, K.; Sebastiani, G. NASH in HIV. Curr. HIV/AIDS Rep. 2020, 17, 601–614. [Google Scholar]
- Davyduke, T.; Tandon, P.; Al-Karaghouli, M.; Abraldes, J.G.; Ma, M. Impact of Implementing a “FIB-4 First” Strategy on a Pathway for Patients With NAFLD Referred From Primary Care. Hepatol. Commun. 2019, 3, 1322–1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaheen, A.A.; Riazi, K.; Medellin, A.; Bhayana, D.; Kaplan, G.G.; Jiang, J.; Park, R.; Schaufert, W.; Burak, K.W.; Sargious, M.; et al. Risk stratification of patients with nonalcoholic fatty liver disease using a case identification pathway in primary care: A cross-sectional study. CMAJ Open 2020, 8, E370–E376. [Google Scholar] [CrossRef]
- Srivastava, A.; Gailer, R.; Tanwar, S.; Trembling, P.; Parkes, J.; Rodger, A.; Suri, D.; Thorburn, D.; Sennett, K.; Morgan, S.; et al. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. J. Hepatol. 2019, 71, 371–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McPherson, S.; Stewart, S.F.; Henderson, E.; Burt, A.D.; Day, C.P. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010, 59, 1265–1269. [Google Scholar] [CrossRef] [Green Version]
- Angulo, P.; Bugianesi, E.; Bjornsson, E.S.; Charatcharoenwitthaya, P.; Mills, P.R.; Barrera, F.; Haflidadottir, S.; Day, C.P.; George, J. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2013, 145, 782–789.e784. [Google Scholar] [CrossRef] [Green Version]
- Guaraldi, G.; Orlando, G.; Zona, S.; Menozzi, M.; Carli, F.; Garlassi, E.; Berti, A.; Rossi, E.; Roverato, A.; Palella, F. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin. Infect. Dis 2011, 53, 1120–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guaraldi, G.; Squillace, N.; Stentarelli, C.; Orlando, G.; D’Amico, R.; Ligabue, G.; Fiocchi, F.; Zona, S.; Loria, P.; Esposito, R.; et al. Nonalcoholic fatty liver disease in HIV-infected patients referred to a metabolic clinic: Prevalence, characteristics, and predictors. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2008, 47, 250–257. [Google Scholar] [CrossRef] [Green Version]
- Sebastiani, G.; Cocciolillo, S.; Mazzola, G.; Malagoli, A.; Falutz, J.; Cervo, A.; Petta, S.; Pembroke, T.; Ghali, P.; Besutti, G.; et al. Application of guidelines for the management of nonalcoholic fatty liver disease in three prospective cohorts of HIV-monoinfected patients. HIV Med. 2020, 21, 96–108. [Google Scholar] [CrossRef]
- Castera, L. Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology 2012, 142, 1293–1302 e1294. [Google Scholar] [CrossRef] [Green Version]
- Eddowes, P.J.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.J.; Paradis, V.; et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef] [Green Version]
- Lemoine, M.; Assoumou, L.; De Wit, S.; Girard, P.M.; Valantin, M.A.; Katlama, C.; Necsoi, C.; Campa, P.; Huefner, A.D.; Schulze Zur Wiesch, J.; et al. Diagnostic Accuracy of Noninvasive Markers of Steatosis, NASH, and Liver Fibrosis in HIV-Monoinfected Individuals at Risk of Nonalcoholic Fatty Liver Disease (NAFLD): Results From the ECHAM Study. J. Acquir. Immune Defic. Syndr. 2019, 80, e86–e94. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Karlas, T.; Petroff, D.; Sasso, M.; Fan, J.G.; Mi, Y.Q.; de Ledinghen, V.; Kumar, M.; Lupsor-Platon, M.; Han, K.H.; Cardoso, A.C.; et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J. Hepatol. 2017, 66, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.L.; Hoofnagle, J.H. Ratio of serum aspartate to alanine aminotransferase in chronic hepatitis. Relationship to cirrhosis. Gastroenterology 1988, 95, 734–739. [Google Scholar] [CrossRef]
- Sattar, N.; Forrest, E.; Preiss, D. Non-alcoholic fatty liver disease. BMJ 2014, 349, g4596. [Google Scholar] [CrossRef] [PubMed]
- Wai, C.T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef]
- Harrison, S.A.; Oliver, D.; Arnold, H.L.; Gogia, S.; Neuschwander-Tetri, B.A. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut 2008, 57, 1441–1447. [Google Scholar] [CrossRef]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef]
- Congly, S.E.; Shaheen, A.A.; Swain, M.G. Modelling the cost effectiveness of non-alcoholic fatty liver disease risk stratification strategies in the community setting. PLoS ONE 2021, 16, e0251741. [Google Scholar] [CrossRef]
- Ma, I.; Lau, C.K.; Ramdas, Z.; Jackson, R.; Naugler, C. Estimated costs of 51 commonly ordered laboratory tests in Canada. Clin. Biochem 2019, 65, 58–60. [Google Scholar] [CrossRef]
- Ingiliz, P.; Valantin, M.A.; Duvivier, C.; Medja, F.; Dominguez, S.; Charlotte, F.; Tubiana, R.; Poynard, T.; Katlama, C.; Lombes, A.; et al. Liver damage underlying unexplained transaminase elevation in human immunodeficiency virus-1 mono-infected patients on antiretroviral therapy. Hepatology 2009, 49, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Lui, G.; Wong, V.W.; Wong, G.L.; Chu, W.C.; Wong, C.K.; Yung, I.M.; Wong, R.Y.; Yeung, S.L.; Yeung, D.K.; Cheung, C.S.; et al. Liver fibrosis and fatty liver in Asian HIV-infected patients. Aliment. Pharm. 2016, 44, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Morse, C.G.; McLaughlin, M.; Matthews, L.; Proschan, M.; Thomas, F.; Gharib, A.M.; Abu-Asab, M.; Orenstein, A.; Engle, R.E.; Hu, X.; et al. Nonalcoholic Steatohepatitis and Hepatic Fibrosis in HIV-1-Monoinfected Adults With Elevated Aminotransferase Levels on Antiretroviral Therapy. Clin. Infect. Dis 2015, 60, 1569–1578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemoine, M.; Barbu, V.; Girard, P.M.; Kim, M.; Bastard, J.P.; Wendum, D.; Paye, F.; Housset, C.; Capeau, J.; Serfaty, L. Altered hepatic expression of SREBP-1 and PPARgamma is associated with liver injury in insulin-resistant lipodystrophic HIV-infected patients. AIDS 2006, 20, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, R.; Lever, R.; Smith, C.; Marshall, N.; Rodger, A.; Bhagani, S.; Tsochatzis, E. Liver test abnormalities in patients with HIV mono-infection: Assessment with simple noninvasive fibrosis markers. Ann. Gastroenterol. 2017, 30, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Patel, P.J.; Rhodes, F.; Srivastava, A.; Patch, D.; Rosenberg, W. Decompensated cirrhosis is the commonest presentation for NAFLD patients undergoing liver transplant assessment. Clin. Med. (London) 2020, 20, 313–318. [Google Scholar] [CrossRef]
- Kirkegaard-Klitbo, D.M.; Bendtsen, F.; Lundgren, J.; Nielsen, S.D.; Benfield, T. Group Cs: Poor Concordance Between Liver Stiffness and Noninvasive Fibrosis Scores in HIV Infection Without Viral Hepatitis. Clin. Gastroenterol. Hepatol. 2019, 18, 3049–3050. [Google Scholar] [CrossRef]
- Lake, J.E.; Trevillyan, J. Impact of Integrase inhibitors and tenofovir alafenamide on weight gain in people with HIV. Curr. Opin. HIV AIDS 2021, 16, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Riazi, K.; Raman, M.; Taylor, L.; Swain, M.G.; Shaheen, A.A. Dietary Patterns and Components in Nonalcoholic Fatty Liver Disease (NAFLD): What Key Messages Can Health Care Providers Offer? Nutrients 2019, 11, 2878. [Google Scholar] [CrossRef] [Green Version]
- Bischoff, J.; Gu, W.; Schwarze-Zander, C.; Boesecke, C.; Wasmuth, J.C.; van Bremen, K.; Dold, L.; Rockstroh, J.K.; Trebicka, J. Stratifying the risk of NAFLD in patients with HIV under combination antiretroviral therapy (cART). EClinicalMedicine 2021, 40, 101116. [Google Scholar] [CrossRef] [PubMed]
- Perazzo, H.; Cardoso, S.W.; Yanavich, C.; Nunes, E.P.; Morata, M.; Gorni, N.; da Silva, P.S.; Cardoso, C.; Almeida, C.; Luz, P.; et al. Predictive factors associated with liver fibrosis and steatosis by transient elastography in patients with HIV mono-infection under long-term combined antiretroviral therapy. J. Int. AIDS Soc. 2018, 21, e25201. [Google Scholar] [CrossRef] [PubMed]
- Guaraldi, G.; Maurice, J.B.; Marzolini, C.; Monteith, K.; Milic, J.; Tsochatzis, E.; Bhagani, S.; Morse, C.G.; Price, J.C.; Ingiliz, P.; et al. New Drugs for NASH and HIV Infection: Great Expectations for a Great Need. Hepatology 2020, 71, 1831–1844. [Google Scholar] [CrossRef] [PubMed]



| LSM ≥ 7.1 kPa (n = 264) | LSM < 7.1 kPa (n = 1485) | p | |
|---|---|---|---|
| Age (years) | 53.1 (9.4) | 49.7 (10.5) | <0.001 |
| Male sex (%) | 203 (76.9) | 1100 (74.1) | 0.333 |
| Ethnicity (%) | |||
| White/Caucasian | 231 (87.5) | 1250 (84.2) | 0.167 |
| Black non-Hispanic | 25 (9.5) | 168 (11.3) | |
| Diabetes (%) | 110 (41.7) | 484 (32.6) | <0.001 |
| BMI (Kg/m2) ° | 27.2 (5.3) | 24.7 (4.0) | <0.001 |
| Time since HIV diagnosis (years) | 18.9 (10.3) | 14.9 (9.6) | <0.001 |
| Undetectable HIV viral load (<40 copies/mL) (%) | 203 (77.0) | 1099 (74.0) | 0.322 |
| CD4 cell count (cells/μL) | 669.3 (347.4) | 708.9 (312.6) | 0.065 |
| Current ART regimen (%) | |||
| NRTIs | 228 (86.4) | 1268 (85.4) | 0.678 |
| NNRTIs | 122 (46.2) | 649 (43.7) | 0.449 |
| Protease inhibitors | 148 (56.1) | 768 (51.7) | 0.192 |
| Integrase inhibitors | 83 (31.4) | 538 (36.2) | 0.134 |
| Past exposure to didanosine (%) | 23 (8.7) | 107 (7.2) | 0.390 |
| ALT (IU/L) | 35.5 (32.3) | 24.5 (16.1) | <0.001 |
| AST (IU/L) | 31.7 (24.2) | 23.1 (10.4) | <0.001 |
| Platelets (109 cells/L) | 202.3 (74.3) | 223.8 (62.3) | <0.001 |
| Albumin (g/dL) | 4.32 (0.55) | 4.37 (0.40) | 0.119 |
| Triglycerides (mmol/L) | 1.96 (1.76) | 1.51 (1.06) | <0.001 |
| Total cholesterol (mmol/L) | 3.58 (1.89) | 2.97 (2.23) | <0.001 |
| HDL (mmol/L) | 1.18 (0.38) | 1.28 (0.39) | <0.001 |
| CAP (dB/m) | 269.2 (61.7) | 230.8 (54.2) | <0.001 |
| AST: ALT ratio | 1.07 (0.65) | 1.09 (0.44) | 0.375 |
| BARD score | 2.20 (1.14) | 2.01 (1.11) | 0.015 |
| NAFLD fibrosis score | −0.84 (1.55) | −1.70 (1.36) | <0.001 |
| FIB-4 | 1.91 (2.00) | 1.20 (0.67) | <0.001 |
| APRI | 0.60 (0.82) | 0.33 (0.20) | <0.001 |
| APRI | FIB-4 | NAFLD Fibrosis Score | BARD Score | AST: ALT Ratio | |
|---|---|---|---|---|---|
| Decrease in TE referral (%) | 86.3 | 63.0 | 51.2 | 24.9 | 26.2 |
| Discordance high LSM/ low biomarker (%) | 11.7 | 11.0 | 8.5 | 15.4 | 19.5 |
| Direct cost of serum biomarker per 100 PWH (CAD) | 1700 | 1700 | 2200 | 1000 | 1000 |
| TE cost saved per 100 PWH (CAD) | 10,788 | 7875 | 6400 | 3113 | 3275 |
| Total direct cost saved per 100 PWH (CAD) | 9088 | 6175 | 4200 | 2113 | 2275 |
| Variable | OR (95% CI) | aOR (95% CI) | p-Value |
|---|---|---|---|
| FIB-4 | |||
| Male sex (yes vs. no) | 0.91 (0.60–1.39) | 0.82 (0.51–1.31) | 0.052 |
| BMI (per Kg/m2) | 1.14 (1.09–1.19) | 1.14 (1.09–1.19) | <0.001 |
| Diabetes (yes vs. no) | 0.83 (0.56–1.28) | 0.77 (0.48–1.23) | 0.270 |
| Triglycerides (per mmol/L) | 1.29 (1.11–1.49) | 1.23 (1.03–1.45) | 0.019 |
| CD4 cell count (per 100 cell/mL) | 0.99 (0.99–1.00) | 0.99 (0.99–1.00) | 0.807 |
| APRI | |||
| Age (per 10 years) | 1.27 (1.09–1.48) | 1.19 (0.99–1.42) | 0.054 |
| Male sex (yes vs. no) | 0.95 (0.67–1.35) | 0.74 (0.50–1.09) | 0.129 |
| BMI (per Kg/m2) | 1.12 (1.08–1.16) | 1.12 (1.08–1.17) | <0.001 |
| Diabetes (yes vs. no) | 1.21 (0.87–1.68) | 1.13 (0.78–1.64) | 0.511 |
| Triglycerides (per mmol/L) | 1.32 (1.17–1.48) | 1.26 (1.11–1.44) | 0.001 |
| CD4 cell count (per 100 cell/mL) | 0.99 (0.99–1.00) | 0.99 (0.99–1.00) | 0.154 |
| CAP ≥248 dB/m (n = 166) | CAP <248 dB/m (n = 98) | p-Value | |
|---|---|---|---|
| Age (years) | 53.6 (8.9) | 52.3 (10.2) | 0.293 |
| Male sex (%) | 135 (81.3) | 68 (69.4) | 0.026 |
| Ethnicity (%) | |||
| White/Caucasian | 139 (83.7) | 92 (93.9) | 0.581 |
| Black non-Hispanic | 17 (10.2) | 8 (8.2) | |
| Diabetes (%) | 66 (60.0) | 44 (40.0) | 0.413 |
| BMI (Kg/m2) ° | 28.7 (4.7) | 24.4 (5.2) | <0.001 |
| Time since HIV diagnosis (years) | 18.5 (9.7) | 19.5 (11.3) | 0.435 |
| Undetectable HIV viral load (<40 copies/mL) (%) | 125 (75.3) | 78 (79.6) | 0.639 |
| CD4 cell count (cells/μL) | 711.3 (656.8) | 599.3 (330.7) | 0.012 |
| Current ART regimen (%) | |||
| NRTIs | 141 (84.9) | 87 (88.8) | 0.380 |
| NNRTIs | 80 (48.3) | 42 (43.9) | 0.401 |
| Protease inhibitors | 101 (60.8) | 62 (63.3) | 0.696 |
| Integrase inhibitors | 48 (28.9) | 35 (35.7) | 0.250 |
| Past exposure to didanosine (%) | 6 (3.6) | 17 (17.3) | <0.001 |
| ALT (IU/L) | 38.0 (34.9) | 31.3 (27.0) | 0.105 |
| AST (IU/L) | 30.7 (23.7) | 33.3 (24.9) | 0.410 |
| Platelets (109 cells/L) | 214.2 (68.9) | 182.1 (78.9) | <0.001 |
| Albumin (g/L) | 43.5 (5.4) | 42.6 (5.7) | 0.233 |
| Triglycerides (mmol/L) | 2.3 (2.1) | 1.4 (0.8) | <0.001 |
| Total cholesterol (mmol/L) | 4.6 (1.1) | 1.9 (1.7) | <0.001 |
| HDL (mmol/L) | 1.1 (0.4) | 1.3 (0.4) | 0.012 |
| AST:ALT ratio | 0.93 (0.34) | 1.29 (0.93) | <0.001 |
| BARD score | 2.19 (1.14) | 2.22 (1.14) | 0.818 |
| NAFLD fibrosis score | −0.95 (1.41) | −0.66 (1.75) | 0.247 |
| FIB-4 | 1.50 (1.11) | 2.61 (2.83) | <0.001 |
| APRI | 0.50 (0.61) | 0.77 (1.07) | 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sebastiani, G.; Milic, J.; Cervo, A.; Saeed, S.; Krahn, T.; Kablawi, D.; Al Hinai, A.S.; Lebouché, B.; Wong, P.; Deschenes, M.; et al. Two-Tier Care Pathways for Liver Fibrosis Associated to Non-Alcoholic Fatty Liver Disease in HIV Mono-Infected Patients. J. Pers. Med. 2022, 12, 282. https://doi.org/10.3390/jpm12020282
Sebastiani G, Milic J, Cervo A, Saeed S, Krahn T, Kablawi D, Al Hinai AS, Lebouché B, Wong P, Deschenes M, et al. Two-Tier Care Pathways for Liver Fibrosis Associated to Non-Alcoholic Fatty Liver Disease in HIV Mono-Infected Patients. Journal of Personalized Medicine. 2022; 12(2):282. https://doi.org/10.3390/jpm12020282
Chicago/Turabian StyleSebastiani, Giada, Jovana Milic, Adriana Cervo, Sahar Saeed, Thomas Krahn, Dana Kablawi, Al Shaima Al Hinai, Bertrand Lebouché, Philip Wong, Marc Deschenes, and et al. 2022. "Two-Tier Care Pathways for Liver Fibrosis Associated to Non-Alcoholic Fatty Liver Disease in HIV Mono-Infected Patients" Journal of Personalized Medicine 12, no. 2: 282. https://doi.org/10.3390/jpm12020282
APA StyleSebastiani, G., Milic, J., Cervo, A., Saeed, S., Krahn, T., Kablawi, D., Al Hinai, A. S., Lebouché, B., Wong, P., Deschenes, M., Gioè, C., Cascio, A., Mazzola, G., & Guaraldi, G. (2022). Two-Tier Care Pathways for Liver Fibrosis Associated to Non-Alcoholic Fatty Liver Disease in HIV Mono-Infected Patients. Journal of Personalized Medicine, 12(2), 282. https://doi.org/10.3390/jpm12020282