The Relationship between Cardiac Magnetic Resonance-Assessed Replacement and Interstitial Fibrosis and Ventricular Arrhythmias in Hypertrophic Cardiomyopathy
Abstract
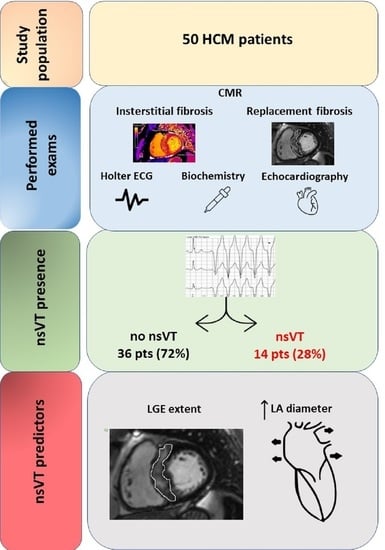
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Cardiac Magnetic Resonance
2.2.1. Assessment of Replacement Fibrosis
2.2.2. Assessment of Interstitial Fibrosis
2.3. Electrocardiographic Examinations
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. nsVT and CMR Data
3.3. Predictor Factors for nsVT
4. Discussion
4.1. nsVT Predictors
4.1.1. Replacement Fibrosis
4.1.2. Left Atrium and nsVT
4.1.3. Interstitial Fibrosis
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Semsarian, C.; Ingles, J.; Maron, M.S.; Maron, B.J. New Perspectives on the Prevalence of Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2015, 65, 1249–1254. [Google Scholar] [CrossRef] [Green Version]
- Dorobantu, L.; Ticulescu, R.; Greavu, M.; Dermengiu, A.; Alexandrescu, M.; Trofin, M. Current Management and Surgical Advances in Patients with Hypertrophic Obstructive Cardiomyopathy. Kardiol. Pol. 2019, 77, 829–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zschirnt, M.; Thul, J.; Akintürk, H.; Valeske, K.; Schranz, D.; Skrzypek, S.; Müller, M.; Jux, C.; Hahn, A.; Rupp, S. Aetiology and 30-Year Long-Term Outcome of Children with Cardiomyopathy Necessitating Heart Transplantation. J. Pers. Med. 2020, 10, 251. [Google Scholar] [CrossRef] [PubMed]
- Marian, A.J.; Braunwald, E. Hypertrophic Cardiomyopathy: Genetics, Pathogenesis, Clinical Manifestations, Diagnosis, and Therapy. Circ. Res. 2017, 121, 749–770. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J. Clinical Course and Management of Hypertrophic Cardiomyopathy. N. Engl. J. Med. 2018, 379, 655–668. [Google Scholar] [CrossRef]
- Maron, B.J.; Rowin, E.J.; Casey, S.A.; Maron, M.S. How Hypertrophic Cardiomyopathy Became a Contemporary Treatable Genetic Disease with Low Mortality: Shaped by 50 Years of Clinical Research and Practice. JAMA Cardiol. 2016, 1, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Maron, B.J.; Maron, M.S. Hypertrophic Cardiomyopathy. Lancet 2013, 381, 242–255. [Google Scholar] [CrossRef]
- Geske, J.B.; Ommen, S.R.; Gersh, B.J. Hypertrophic Cardiomyopathy: Clinical Update. JACC Heart Fail. 2018, 6, 364–375. [Google Scholar] [CrossRef]
- Taylor, A.J.; Salerno, M.; Dharmakumar, R.; Jerosch-Herold, M. T1 Mapping Basic Techniques and Clinical Applications. JACC Cardiovasc. Imaging 2016, 9, 67–81. [Google Scholar] [CrossRef] [Green Version]
- Bacmeister, L.; Schwarzl, M.; Warnke, S.; Stoffers, B.; Blankenberg, S.; Westermann, D.; Lindner, D. Inflammation and Fibrosis in Murine Models of Heart Failure; Springer: Berlin/Heidelberg, Germany, 2019; Volume 114. [Google Scholar] [CrossRef]
- Rai, V.; Sharma, P.; Agrawal, S.; Agrawal, D.K. Relevance of Mouse Models of Cardiac Fibrosis and Hypertrophy in Cardiac Research. Mol. Cell. Biochem. 2017, 424, 123–145. [Google Scholar] [CrossRef]
- Cooper, L.T.; Baughman, K.L.; Feldman, A.M.; Frustaci, A.; Jessup, M.; Kuhl, U.; Levine, G.N.; Narula, J.; Starling, R.C.; Towbin, J.; et al. The Role of Endomyocardial Biopsy in the Management of Cardiovascular Disease: A Scientific Statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. Eur. Heart J. 2007, 28, 3076–3093. [Google Scholar] [CrossRef] [PubMed]
- Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Lafont, A.; Limongelli, G.; Mahrholdt, H.; et al. European Society of Cardiology Guidelines on Diagnosis and Management of Hypertrophic Cardiomyopathy. Eur. Heart J. 2014, 35, 2733–2779. [Google Scholar] [PubMed]
- Lang, R.M.; Badano, L.P.; Victor, M.A.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical Recommendations for Cardiovascular Magnetic Resonance Mapping of T1, T2, T2 and Extracellular Volume: A Consensus Statement by the Society for Cardiovascular Magnetic Resonance (SCMR) Endorsed by the European Association for Cardiovascular Imagin. J. Cardiovasc. Magn. Reson. 2017, 19, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boas, R.; Thune, J.J.; Pehrson, S.; Køber, L.; Nielsen, J.C.; Videbæk, L.; Haarbo, J.; Korup, E.; Bruun, N.E.; Brandes, A.; et al. Prevalence and Prognostic Association of Ventricular Arrhythmia in Non-Ischaemic Heart Failure Patients: Results from the DANISH Trial. Europace 2021, 23, 587–595. [Google Scholar] [CrossRef]
- Rudolph, A.; Abdel-Aty, H.; Bohl, S.; Boyé, P.; Zagrosek, A.; Dietz, R.; Schulz-Menger, J. Noninvasive Detection of Fibrosis Applying Contrast-Enhanced Cardiac Magnetic Resonance in Different Forms of Left Ventricular Hypertrophy. Relation to Remodeling. J. Am. Coll. Cardiol. 2009, 53, 284–291. [Google Scholar] [CrossRef] [Green Version]
- Moon, J.C.C.; Reed, E.; Sheppard, M.N.; Elkington, A.G.; Ho, S.Y.; Burke, M.; Petrou, M.; Pennell, D.J. The Histologic Basis of Late Gadolinium Enhancement Cardiovascular Magnetic Resonance in Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2004, 43, 2260–2264. [Google Scholar] [CrossRef]
- Choudhury, L.; Mahrholdt, H.; Wagner, A.; Choi, K.M.; Elliott, M.D.; Klocke, F.J.; Bonow, R.O.; Judd, R.M.; Kim, R.J. Myocardial Scarring in Asymptomatic or Mildly Symptomatic Patients with Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2002, 40, 2156–2164. [Google Scholar] [CrossRef] [Green Version]
- Adabag, A.S.; Maron, B.J.; Appelbaum, E.; Harrigan, C.J.; Buros, J.L.; Gibson, C.M.; Lesser, J.R.; Hanna, C.A.; Udelson, J.E.; Manning, W.J.; et al. Occurrence and Frequency of Arrhythmias in Hypertrophic Cardiomyopathy in Relation to Delayed Enhancement on Cardiovascular Magnetic Resonance. J. Am. Coll. Cardiol. 2008, 51, 1369–1374. [Google Scholar] [CrossRef] [Green Version]
- Prinz, C.; Schwarz, M.; Ilic, I.; Laser, K.T.; Lehmann, R.; Prinz, E.M.; Bitter, T.; Vogt, J.; Van Buuren, F.; Bogunovic, N.; et al. Myocardial Fibrosis Severity on Cardiac Magnetic Resonance Imaging Predicts Sustained Arrhythmic Events in Hypertrophic Cardiomyopathy. Can. J. Cardiol. 2013, 29, 358–363. [Google Scholar] [CrossRef]
- Ommen, S.R.; Mital, S.; Burke, M.A.; Day, S.M.; Deswal, A.; Elliott, P.; Evanovich, L.L.; Hung, J.; Joglar, J.A.; Kantor, P.; et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients with Hypertrophic Cardiomyopathy: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2020, 76, 3022–3055. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.H.; Maron, B.J.; Olivotto, I.; Pencina, M.J.; Assenza, G.E.; Haas, T.; Lesser, J.R.; Gruner, C.; Crean, A.M.; Rakowski, H.; et al. Prognostic Value of Quantitative Contrast-Enhanced Cardiovascular Magnetic Resonance for the Evaluation of Sudden Death Risk in Patients with Hypertrophic Cardiomyopathy. Circulation 2014, 130, 484–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, Z.; Yao, J.; Chan, R.H.; He, J.; Yang, X.; Zhou, Y.; He, Y. Prognostic Value of LGE-CMR in HCM: A Meta-Analysis. JACC Cardiovasc. Imaging 2016, 9, 1392–1402. [Google Scholar] [CrossRef]
- Weissler-Snir, A.; Hindieh, W.; Spears, D.A.; Adler, A.; Rakowski, H.; Chan, R.H. The Relationship between the Quantitative Extent of Late Gadolinium Enhancement and Burden of Nonsustained Ventricular Tachycardia in Hypertrophic Cardiomyopathy: A Delayed Contrast-Enhanced Magnetic Resonance Study. J. Cardiovasc. Electrophysiol. 2019, 30, 651–657. [Google Scholar] [CrossRef]
- Briasoulis, A.; Mallikethi-Reddy, S.; Palla, M.; Alesh, I.; Afonso, L. Myocardial Fibrosis on Cardiac Magnetic Resonance and Cardiac Outcomes in Hypertrophic Cardiomyopathy: A Meta-Analysis. Heart 2015, 101, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Maron, M.S.; Appelbaum, E.; Harrigan, C.J.; Buros, J.; Gibson, C.M.; Hanna, C.; Lesser, J.R.; Udelson, J.E.; Manning, W.J.; Maron, B.J. Clinical Profile and Significance of Delayed Enhancement in Hypertrophic Cardiomyopathy. Circ. Heart Fail. 2008, 1, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Green, J.J.; Berger, J.S.; Kramer, C.M.; Salerno, M. Prognostic Value of Late Gadolinium Enhancement in Clinical Outcomes for Hypertrophic Cardiomyopathy. JACC Cardiovasc. Imaging 2012, 5, 370–377. [Google Scholar] [CrossRef] [Green Version]
- Ismail, T.F.; Jabbour, A.; Gulati, A.; Mallorie, A.; Raza, S.; Cowling, T.E.; Das, B.; Khwaja, J.; Alpendurada, F.D.; Wage, R.; et al. Role of Late Gadolinium Enhancement Cardiovascular Magnetic Resonance in the Risk Stratification of Hypertrophic Cardiomyopathy. Heart 2014, 100, 1851–1858. [Google Scholar] [CrossRef]
- O’Hanlon, R.; Grasso, A.; Roughton, M.; Moon, J.C.; Clark, S.; Wage, R.; Webb, J.; Kulkarni, M.; Dawson, D.; Sulaibeekh, L.; et al. Prognostic Significance of Myocardial Fibrosis in Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 867–874. [Google Scholar] [CrossRef] [Green Version]
- McLellan, A.J.A.; Ellims, A.H.; Prabhu, S.; Voskoboinik, A.; Iles, L.M.; Hare, J.L.; Kaye, D.M.; MacCiocca, I.; Mariani, J.A.; Kalman, J.M.; et al. Diffuse Ventricular Fibrosis on Cardiac Magnetic Resonance Imaging Associates with Ventricular Tachycardia in Patients with Hypertrophic Cardiomyopathy. J. Cardiovasc. Electrophysiol. 2016, 27, 571–580. [Google Scholar] [CrossRef]
- Avanesov, M.; Münch, J.; Weinrich, J.; Well, L.; Säring, D.; Stehning, C.; Tahir, E.; Bohnen, S.; Radunski, U.K.; Muellerleile, K.; et al. Prediction of the Estimated 5-Year Risk of Sudden Cardiac Death and Syncope or Non-Sustained Ventricular Tachycardia in Patients with Hypertrophic Cardiomyopathy Using Late Gadolinium Enhancement and Extracellular Volume CMR. Eur. Radiol. 2017, 27, 5136–5145. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.; Collins, J.D.; Ogele, E.; Murtagh, G.; Carr, J.C.; Bonow, R.O.; Choudhury, L. Relation of Late Gadolinium Enhancement and Extracellular Volume Fraction to Ventricular Arrhythmias in Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2020, 131, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Mentias, A.; Raeisi-Giglou, P.; Smedira, N.G.; Feng, K.; Sato, K.; Wazni, O.; Kanj, M.; Flamm, S.D.; Thamilarasan, M.; Popovic, Z.B.; et al. Late Gadolinium Enhancement in Patients With Hypertrophic Cardiomyopathy and Preserved Systolic Function. J. Am. Coll. Cardiol. 2018, 72, 857–870. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, C.; Jichi, F.; Pavlou, M.; Monserrat, L.; Anastasakis, A.; Rapezzi, C.; Biagini, E.; Gimeno, J.R.; Limongelli, G.; McKenna, W.J.; et al. A Novel Clinical Risk Prediction Model for Sudden Cardiac Death in Hypertrophic Cardiomyopathy (HCM Risk-SCD). Eur. Heart J. 2014, 35, 2010–2020. [Google Scholar] [CrossRef]
- Spirito, P.; Autore, C.; Rapezzi, C.; Bernabò, P.; Badagliacca, R.; Maron, M.S.; Bongioanni, S.; Coccolo, F.; Estes, N.A.M.; Barillà, C.S.; et al. Syncope and Risk of Sudden Death in Hypertrophic Cardiomyopathy. Circulation 2009, 119, 1703–1710. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.; Park, C.H.; Kim, Y.; Kim, J.Y.; Min, P.K.; Yoon, Y.W.; Lee, K.A.; Lee, B.K.; Hong, B.K.; Kim, T.H.; et al. Burden of Premature Ventricular Contractions beyond Nonsustained Ventricular Tachycardia Is Related to the Myocardial Extracellular Space Expansion in Patients with Hypertrophic-Cardiomyopathy. Clin. Cardiol. 2020, 43, 1317–1325. [Google Scholar] [CrossRef]
- Mirelis, J.G.; Sánchez-González, J.; Zorio, E.; Ripoll-Vera, T.; Salguero-Bodes, R.; Filgueiras-Rama, D.; González-López, E.; Gallego-Delgado, M.; Fernández-Jiménez, R.; Soleto, M.J.; et al. Myocardial Extracellular Volume Is Not Associated With Malignant Ventricular Arrhythmias in High-Risk Hypertrophic Cardiomyopathy. Rev. Española Cardiol. (Engl. Ed.) 2017, 70, 933–940. [Google Scholar] [CrossRef]
- Karamitsos, T.D.; Arvanitaki, A.; Karvounis, H.; Neubauer, S.; Ferreira, V.M. Myocardial Tissue Characterization and Fibrosis by Imaging. JACC Cardiovasc. Imaging 2020, 13, 1221–1234. [Google Scholar] [CrossRef]


| Without nsVT (n = 36) | With nsVT (n = 14) | p-Value | |
|---|---|---|---|
| Age (years) | 48 (27.5) | 54 (17) | 0.3 |
| Sex—male (n, %) | 24 (66.7%) | 11 (78.6%) | 0.41 |
| BMI (kg/m2) | 29 ± 5.1 | 32.7 ± 6.6 | 0.042 |
| LVOTO (n, %) | 13 (36.1%) | 6 (42.9%) | 0.66 |
| Diabetes mellitus (n, %) | 3 (8.3%) | 3 (21.4%) | 0.2 |
| Coronary artery disease (n, %) | 5 (13.9%) | 3 (21.4%) | 0.51 |
| Hypertension (n, %) | 20 (55.6%) | 9 (64.3%) | 0.57 |
| Atrial fibrillation (n, %) | 3 (8.3%) | 3 (21.4%) | 0.2 |
| Dyslipidaemia (n, %) | 14 (38.9%) | 10 (71.4%) | 0.039 |
| Syncope (n, %) | 5 (13.9%) | 2 (14.3%) | 0.97 |
| Family history of SCD (n, %) | 4 (11.1%) | 1 (7.1%) | 0.67 |
| Estimated 5-year risk of SCD (%) | 2.09 (1.76) | 5.8 (3.3) | <0.0001 |
| NYHA class | 1 (1) | 2 (1) | 0.28 |
| SBP (mmHg) | 127.8 ± 21.8 | 140.2 ± 18.17 | 0.07 |
| 6MWT-distance (m) | 441 ± 121.1 | 411.2 ± 119.3 | 0.5 |
| 6MWT-Borg scale | 3 (4) | 2 (4.5) | 0.9 |
| LVEDd/BSA (mm/m2) | 22.7 ±3.4 | 21.8 ± 3.6 | 0.43 |
| MWT (mm) | 19 (5.5) | 21 (4) | 0.098 |
| LVEF (%) | 65 (10) | 68 (15) | 0.34 |
| LA (mm) | 41 (7) | 46 (11) | 0.0007 |
| LAVI (mL/m2) | 39.5 (19.9) | 59 (31.9) | 0.01 |
| E/e’ | 10 (5.6) | 12.6 (8.6) | 0.046 |
| Max. LVOT gradient (mmHg) | 21 (39.5) | 37.5 (80) | 0.47 |
| RVSP (mmHg) | 23.5 (11.5) | 23 (12) | 0.77 |
| Hb (g/dL) | 14.4 (1.9) | 14.7 (1.9) | 0.85 |
| Hct (%) | 41.6 ± 4.5 | 42.8 ± 3.8 | 0.3 |
| hsTnT (ng/mL) | 0.015 (0.017) | 0.018 (0.014) | 0.46 |
| NT-proBNP (pg/mL) | 444.5 (946.5) | 671 (978) | 0.33 |
| BB (n, %) | 31 (86.1%) | 12 (85.7%) | 0.97 |
| Diltiazem/verapamil (n, %) | 5 (13.9%) | 2 (14.3%) | 0.97 |
| ASA (n, %) | 7 (19.4%) | 1 (7.1%) | 0.29 |
| ACEi/ARB (n, %) | 15 (41.7%) | 7 (50%) | 0.59 |
| MRA (n, %) | 8 (22.2%) | 6 (42.9%) | 0.14 |
| Loop diuretics (n, %) | 10 (27.8%) | 4 (28.6%) | 0.96 |
| Amiodarone (n, %) | 1 (2.8%) | 1 (7.14%) | 0.48 |
| OAC (n, %) | 3 (8.3%) | 3 (21.4%) | 0.2 |
| Statins (n, %) | 15 (41.7%) | 5 (35.7%) | 0.67 |
| Without nsVT (n = 34) | With nsVT (n = 14) | p-Value | |
|---|---|---|---|
| LVEF (%) | 69 (11) | 69.5 (10) | 0.4 |
| Svi (mL/m2) | 51.5 ± 10.2 | 56.6 ± 9.7 | 0.1 |
| LV mass (g) | 190.9 ± 55 | 217.3 ± 46.2 | 0.13 |
| LGE (n, %) | 24 (66.7%) | 13 (92.9%) | 0.074 |
| %LGE (%) | 2.69 (5.47) | 8.1 (7.36) | 0.002 |
| T1 native blood (ms) | 1852.7 (111) | 1842.7 (94.3) | 0.5 |
| T1 post-contrast blood (ms) | 302 (44.7) | 326.7 (57.3) | 0.87 |
| T1 native septal (ms) | 1264 (91) | 1305.6 (91.8) | 0.43 |
| T1 native global (ms) | 1258.9 + 70.5 | 1275.1 ± 59.6 | 0.45 |
| T1 post-contrast septal (ms) | 489.5 (64) | 487.5 (92) | 0.52 |
| T1 post-contrast global(ms) | 471.2 ± 57 | 468.2 ± 53.4 | 0.87 |
| ECV septal (%) | 26.5 (5.2) | 27.9 (5.4) | 0.099 |
| ECV global (%) | 28.1 (6.2) | 28.1 (4.8) | 0.6 |
| Parameter | Univariable OR (95% CI) p-Value | Multivariable OR (95% CI) p-Value | ||
|---|---|---|---|---|
| Dyslipidemia | 3.93 (0.99–15.5) | 0.05 | - | - |
| BMI | 1.12 (0.997–1.269) | 0.05 | - | - |
| E/E’ | 1.21 (1.02–1.43) | 0.024 | 1.19 (0.95–1.48) | 0.1 |
| LA | 1.2 (1.06–1.36) | 0.004 | 1.19 (1.03–1.38) | 0.016 |
| LGE extent | 1.17 (1.02–1.35) | 0.02 | 1.2 (1.02–1.4) | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karabinowska-Małocha, A.; Dziewięcka, E.; Banyś, P.; Urbańczyk-Zawadzka, M.; Krupiński, M.; Mielnik, M.; Łach, J.; Budkiewicz, A.; Podolec, P.; Żydzik, Ł.; et al. The Relationship between Cardiac Magnetic Resonance-Assessed Replacement and Interstitial Fibrosis and Ventricular Arrhythmias in Hypertrophic Cardiomyopathy. J. Pers. Med. 2022, 12, 294. https://doi.org/10.3390/jpm12020294
Karabinowska-Małocha A, Dziewięcka E, Banyś P, Urbańczyk-Zawadzka M, Krupiński M, Mielnik M, Łach J, Budkiewicz A, Podolec P, Żydzik Ł, et al. The Relationship between Cardiac Magnetic Resonance-Assessed Replacement and Interstitial Fibrosis and Ventricular Arrhythmias in Hypertrophic Cardiomyopathy. Journal of Personalized Medicine. 2022; 12(2):294. https://doi.org/10.3390/jpm12020294
Chicago/Turabian StyleKarabinowska-Małocha, Aleksandra, Ewa Dziewięcka, Paweł Banyś, Małgorzata Urbańczyk-Zawadzka, Maciej Krupiński, Małgorzata Mielnik, Jacek Łach, Aleksandra Budkiewicz, Piotr Podolec, Łukasz Żydzik, and et al. 2022. "The Relationship between Cardiac Magnetic Resonance-Assessed Replacement and Interstitial Fibrosis and Ventricular Arrhythmias in Hypertrophic Cardiomyopathy" Journal of Personalized Medicine 12, no. 2: 294. https://doi.org/10.3390/jpm12020294
APA StyleKarabinowska-Małocha, A., Dziewięcka, E., Banyś, P., Urbańczyk-Zawadzka, M., Krupiński, M., Mielnik, M., Łach, J., Budkiewicz, A., Podolec, P., Żydzik, Ł., Wiśniowska-Śmiałek, S., Holcman, K., Kostkiewicz, M., & Rubiś, P. (2022). The Relationship between Cardiac Magnetic Resonance-Assessed Replacement and Interstitial Fibrosis and Ventricular Arrhythmias in Hypertrophic Cardiomyopathy. Journal of Personalized Medicine, 12(2), 294. https://doi.org/10.3390/jpm12020294