Pharmacogenomics and Drug-Induced Phenoconversion Informed Medication Safety Review in the Management of Pain Control and Quality of Life: A Case Report
Abstract
:
1. Introduction
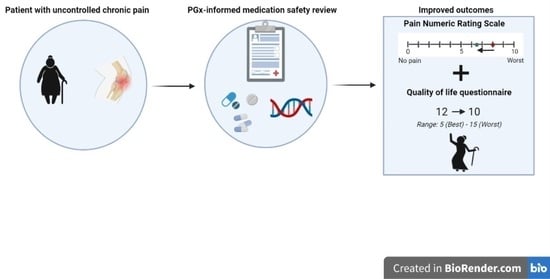
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Tinnirello, A.; Mazzoleni, S.; Santi, C. Chronic Pain in the Elderly: Mechanisms and Distinctive Features. Biomolecules 2021, 11, 1256. [Google Scholar] [CrossRef] [PubMed]
- Ko, T.M.; Wong, C.S.; Wu, J.Y.; Chen, Y.T. Pharmacogenomics for personalized pain medicine. Acta Anaesthesiol. Taiwan 2016, 54, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Crews, K.R.; Monte, A.A.; Huddart, R.; Caudle, K.E.; Kharasch, E.D.; Gaedigk, A.; Dunnenberger, H.M.; Leeder, J.S.; Callaghan, J.T.; Samer, C.F.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline for CYP2D6, OPRM1, and COMT Genotypes and Select Opioid Therapy. Clin. Pharmacol. Ther. 2021, 110, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, S.; Polasek, T.M.; Sheffield, L.J.; Huppert, D.; Kirkpatrick, C.M.J. Quantifying the Impact of Phenoconversion on Medications with Actionable Pharmacogenomic Guideline Recommendations in an Acute Aged Persons Mental Health Setting. Front. Psychiatry 2021, 12, 724170. [Google Scholar] [CrossRef] [PubMed]
- Hicks, J.K.; Dunnenberger, H.M.; Gumpper, K.F.; Haidar, C.E.; Hoffman, J.M. Integrating pharmacogenomics into electronic health records with clinical decision support. Am. J. Health Syst. Pharm. 2016, 73, 1967–1976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bain, K.T.; Knowlton, C.H.; Turgeon, J. Medication Risk Mitigation: Coordinating and Collaborating with Health Care Systems, Universities, and Researchers to Facilitate the Design and Execution of Practice-Based Research. Clin. Geriatr. Med. 2017, 33, 257–281. [Google Scholar] [CrossRef] [PubMed]
- Bankes, D.L.; Jin, H.; Finnel, S.; Michaud, V.; Knowlton, C.H.; Turgeon, J.; Stein, A. Association of a Novel Medication Risk Score with Adverse Drug Events and Other Pertinent Outcomes Among Participants of the Programs of All-Inclusive Care for the Elderly. Pharmacy 2020, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Michaud, V.; Smith, M.K.; Bikmetov, R.; Dow, P.; Johnson, J.; Stein, A.; Finnel, S.; Jin, H.; Turgeon, J. Association of the MedWise Risk Score with health care outcomes. Am. J. Manag. Care 2021, 27, S280–S291. [Google Scholar] [CrossRef] [PubMed]
- McGeeney, B.E. Pharmacological management of neuropathic pain in older adults: An update on peripherally and centrally acting agents. J. Pain Symptom Manag. 2009, 38, S15–S27. [Google Scholar] [CrossRef] [PubMed]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2020, 72, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Long, T.; Cristofoletti, R.; Cicali, B.; Michaud, V.; Dow, P.; Turgeon, J.; Schmidt, S. Physiologically Based Pharmacokinetic Modeling to Assess the Impact of CYP2D6-Mediated Drug-Drug Interactions on Tramadol and O-Desmethyltramadol Exposures via Allosteric and Competitive Inhibition. J. Clin. Pharmacol. 2022, 62, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Viswanath, O.; Saadabadi, A. Buprenorphine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Vadivelu, N.; Hines, R.L. Management of chronic pain in the elderly: Focus on transdermal buprenorphine. Clin. Interv. Aging. 2008, 3, 421–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tylutki, Z.; Mendyk, A.; Polak, S. Physiologically based pharmacokinetic-quantitative systems toxicology and safety (PBPK-QSTS) modeling approach applied to predict the variability of amitriptyline pharmacokinetics and cardiac safety in populations and in individuals. J. Pharm. Pharm. 2018, 45, 663–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hicks, J.K.; Swen, J.J.; Thorn, C.F.; Sangkuhl, K.; Kharasch, E.D.; Ellingrod, V.L.; Skaar, T.C.; Müller, D.J.; Gaedigk, A.; Stingl, J.C. Clinical Pharmacogenetics Implementation Consortium guideline for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants. Clin. Pharmacol. Ther. 2013, 93, 402–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McHugh, B.; Krishnadas, R. Guide to safely withdrawing antidepressants in primary care. Prescriber 2011, 22, 36–40. [Google Scholar] [CrossRef]
- Zvyaga, T.; Chang, S.Y.; Chen, C.; Yang, Z.; Vuppugalla, R.; Hurley, J.; Thorndike, D.; Wagner, A.; Chimalakonda, A.; Rodrigues, A.D. Evaluation of six proton pump inhibitors as inhibitors of various human cytochromes P450: Focus on cytochrome P450 2C19. Drug Metab. Dispos. 2012, 40, 1698–1711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Condition | Medication | Dose | Directions |
|---|---|---|---|
| Anxiety | Hydroxyzine | 50 mg | 1 tablet at bedtime |
| Alprazolam | 0.5 mg | 1 tablet as needed | |
| Atrial fibrillation | Diltiazem | 120 mg | 1 tablet in the morning |
| Circulation | Aspirin | 81 mg | 1 tablet in the morning |
| Apixaban | 5 mg | 1 tablet in the morning and evening | |
| COPD | Albuterol | 90 mcg | 2 puffs every 6 h as needed |
| Ipratropium/albuterol | 0.5 mg–3 mg | 1 vial via nebulizer four times daily | |
| Tiotropium | 1.25 mcg | 2 puffs once daily | |
| Epilepsy | Phenytoin | 100 mg | 1 tablet in the morning and bedtime |
| GERD | Omeprazole | 40 mg | 1 tablet in the morning |
| Hyperlipidemia | Atorvastatin | 40 mg | 1 tablet in the morning |
| Hypertension | Carvedilol | 6.25 mg | 1 tablet in the morning and bedtime |
| Hypokalemia | Potassium chloride | 20 mEq | 1 tablet in the morning |
| Hypothyroidism | Levothyroxine | 25 mcg | 1 tablet in the morning |
| Ischemic cardiomyopathy | Furosemide | 80 mg | 1 tablet once daily |
| Nitroglycerin | 0.4 mg | 1 tablet every 5 min as needed | |
| Sotalol | 120 mg | 1 tablet in the morning and evening | |
| Neuropathy | Gabapentin | 100 mg | 1 capsule in the morning, evening and bedtime |
| Pregabalin | 150 mg | 1 capsule in the morning and evening | |
| Duloxetine | 60 mg | 1 capsule in the morning | |
| Amitriptyline | 75 mg | 1 tablet at bedtime | |
| Nutrient deficiency | Multivitamin | N/A | 1 tablet in the morning |
| Osteoarthritis | Tramadol | 50 mg | 1 tablet in the morning, evening, and bedtime |
| Osteoporosis | Alendronate | 70 mg | 1 tablet once a week |
| Gene | Genotype | Phenotype Summary |
|---|---|---|
| CYP2C19 | *1|*2 | Intermediate Metabolizer |
| CYP2D6 | *2A|*9 | Normal Metabolizer |
| CYP2B6 | *1|*5 | Normal Metabolizer |
| CYP2C9 | *1|*1 | Normal Metabolizer |
| SLCO1B1 | *1B|*1B | Normal Function |
| Substance | CYP1A2 | CYP2B6 | CYP2C9 NM → pRM | CYP2C19 IM → pPM | CYP2D6 NM → pIM | CYP3A4 | |||
|---|---|---|---|---|---|---|---|---|---|
| Alprazolam | |||||||||
| Amitriptyline | |||||||||
| Apixaban | |||||||||
| Atorvastatin | |||||||||
| Carvedilol | |||||||||
| Diltiazem | |||||||||
| Duloxetine | |||||||||
| Hydroxyzine | |||||||||
| Omeprazole | |||||||||
| Phenytoin | |||||||||
| Tramadol | * | ||||||||
| Affinity Strengths | Weak Substrate | Medium Substrate | Strong Substrate | Inhibitor | Inducer | ||||
| Medication | Pharmacist’s Recommendation | Implementation |
|---|---|---|
| Tramadol 50 mg | Discontinue tramadol and utilize a non-CYP2D6 opioid | Tramadol discontinued and buprenorphine transdermal patch 5 mcg/h weekly initiated |
| Amitriptyline 75 mg | Taper off amitriptyline to mitigate risk of ADEs and pharmacotherapy failure | Amitriptyline 50 mg for 1 week, 25 mg for 1 week, then discontinued |
| Omeprazole 40 mg | Switch to pantoprazole 40 mg to mitigate non-competitive inhibition at CYP2C19 | Switched to pantoprazole 40 mg |
| Amitriptyline 75 mg, Furosemide 80 mg, Hydroxyzine 50 mg, Omeprazole 40 mg, Sotalol 120 mg, Tramadol 50 mg | Re-evaluate the need for QT-prolonging medications and obtain ECG | Will monitor ECG |
| Atorvastatin 40 mg | Switch to pravastatin to mitigate drug interaction with phenytoin | Switched to pravastatin 40 mg |
| Pregabalin 150 mg and Gabapentin 100 mg | Utilize either pregabalin or gabapentin to mitigate sedative burden | Gabapentin discontinued and pregabalin dose increased from 150 mg to 225 mg |
| Alprazolam 0.5 mg | Switch to lorazepam to mitigate sedative burden and drug interaction at CYP3A4 | Patient declined |
| Hydroxyzine 50 mg | Taper off hydroxyzine to mitigate anticholinergic and sedative burden | Patient declined |
| Diltiazem 120 mg, Carvedilol 6.25 mg, Sotalol 120 mg | Consult cardiology to evaluate appropriateness of cardiovascular drug regimen | Cardiology consulted |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhn, S.; Amin, N.S.; Bardolia, C.; Del Toro-Pagán, N.; Pizzolato, K.; Thacker, D.; Turgeon, J.; Tomaino, C.; Michaud, V. Pharmacogenomics and Drug-Induced Phenoconversion Informed Medication Safety Review in the Management of Pain Control and Quality of Life: A Case Report. J. Pers. Med. 2022, 12, 974. https://doi.org/10.3390/jpm12060974
Muhn S, Amin NS, Bardolia C, Del Toro-Pagán N, Pizzolato K, Thacker D, Turgeon J, Tomaino C, Michaud V. Pharmacogenomics and Drug-Induced Phenoconversion Informed Medication Safety Review in the Management of Pain Control and Quality of Life: A Case Report. Journal of Personalized Medicine. 2022; 12(6):974. https://doi.org/10.3390/jpm12060974
Chicago/Turabian StyleMuhn, Selina, Nishita Shah Amin, Chandni Bardolia, Nicole Del Toro-Pagán, Katie Pizzolato, David Thacker, Jacques Turgeon, Crystal Tomaino, and Veronique Michaud. 2022. "Pharmacogenomics and Drug-Induced Phenoconversion Informed Medication Safety Review in the Management of Pain Control and Quality of Life: A Case Report" Journal of Personalized Medicine 12, no. 6: 974. https://doi.org/10.3390/jpm12060974
APA StyleMuhn, S., Amin, N. S., Bardolia, C., Del Toro-Pagán, N., Pizzolato, K., Thacker, D., Turgeon, J., Tomaino, C., & Michaud, V. (2022). Pharmacogenomics and Drug-Induced Phenoconversion Informed Medication Safety Review in the Management of Pain Control and Quality of Life: A Case Report. Journal of Personalized Medicine, 12(6), 974. https://doi.org/10.3390/jpm12060974