Allergic Asthma in the Era of Personalized Medicine
Abstract
:1. Introduction
2. Definition
3. Clinical Characteristics
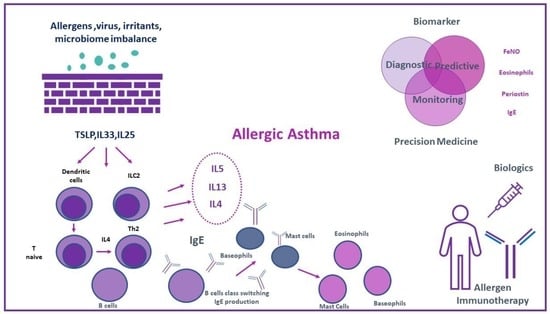
4. Pathogenic Mechanisms in Allergic Asthma
5. Biomarkers
5.1. Total and Specific Immunoglobulin E (IgE)
5.2. Fractional Exhaled Nitric Oxide (FeNO)
5.3. Eosinophils
5.4. Serum Periostin
6. Treatment
6.1. Therapeutic Interventions in Asthma: Standard of Care
6.2. Environmental Interventions
6.3. Corticosteroids
6.4. Allergen Immunotherapy
6.5. Biologic Agents
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Ramadan, A.A.; Gaffin, J.M.; Israel, E.; Phipatanakul, W. Asthma and Corticosteroid Responses in Childhood and Adult Asthma. Clin. Chest Med. 2019, 40, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Settipane, R.A.; Kreindler, J.L.; Chung, Y.; Tkacz, J. Evaluating direct costs and productivity losses of patients with asthma receiving GINA 4/5 therapy in the United States. Ann. Allergy Asthma Immunol. 2019, 123, 564–572.e3. [Google Scholar] [CrossRef] [Green Version]
- Yaghoubi, M.; Adibi, A.; Safari, A.; FitzGerald, J.M.; Sadatsafavi, M. The Projected Economic and Health Burden of Uncontrolled Asthma in the United States. Am. J. Respir. Crit. Care Med. 2019, 200, 1102–1112. [Google Scholar] [CrossRef]
- Global Initiative for Asthma. 2021 GINA Report, Global Management for Asthma Management and Prevention (2021 Update). Available online: https://ginasthma.org/gina-reports (accessed on 4 June 2022).
- Wenzel, S.E. Severe Adult Asthmas: Integrating Clinical Features, Biology, and Therapeutics to Improve Outcomes. Am. J. Respir. Crit. Care Med. 2021, 203, 809–821. [Google Scholar] [CrossRef]
- Wenzel, S.E. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 2012, 18, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Johansson, S.G.; Hourihane, J.O.; Bousquet, J.; Bruijnzeel-Koomen, C.; Dreborg, S.; Haahtela, T.; Kowalski, M.L.; Mygind, N.; Ring, J.; van Cauwenberge, P.; et al. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy 2001, 56, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Schatz, M.; Rosenwasser, L. The allergic asthma phenotype. J. Allergy Clin. Immunol. Pract. 2014, 2, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Romanet-Manent, S.; Charpin, D.; Magnan, A.; Lanteaume, A.; Vervloet, D. Allergic vs. nonallergic asthma: What makes the difference? Allergy 2002, 57, 607–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genuneit, J.; Seibold, A.M.; Apfelbacher, C.J.; Konstantinou, G.N.; Koplin, J.J.; La Grutta, S.; Logan, K.; Perkin, M.R.; Flohr, C. Overview of systematic reviews in allergy epidemiology. Allergy 2017, 72, 849–856. [Google Scholar] [CrossRef] [Green Version]
- Ring, J.; Jutel, M.; Papadopoulos, N.; Pfaar, O.; Akdis, C. Provocative proposal for a revised nomenclature for allergy and other hypersensitivity diseases. Allergy 2018, 73, 1939–1940. [Google Scholar] [CrossRef] [Green Version]
- Agache, I.; Akdis, C.A.; Akdis, M.; Canonica, G.W.; Casale, T.; Chivato, T.; Corren, J.; Chu, D.K.; Del Giacco, S.; Eiwegger, T.; et al. EAACI Biologicals Guidelines-Recommendations for severe asthma. Allergy 2021, 76, 14–44. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Asthma. Difficult-to-Treat and Severe Asthma in Adolescent and Adult Patients: Diagnosis and Management. A GINA Pocket Guide for Health Professionals. 2019. Available online: https://ginasthma.org/wpcontent/uploads/2019/04/GINA-Severe-asthma-Pocket-Guide-v2.0-wms-1.pdf (accessed on 1 June 2022).
- Miranda, C.; Busacker, A.; Balzar, S.; Trudeau, J.; Wenzel, S.E. Distinguishing severe asthma phenotypes: Role of age at onset and eosinophilic inflammation. J. Allergy Clin. Immunol. 2004, 113, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.C.; Meyers, D.A.; Wenzel, S.E.; Teague, W.G.; Li, H.; Li, X.; D’Agostino, R., Jr.; Castro, M.; Curran-Everett, D.; Fitzpatrick, A.M.; et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am. J. Respir. Crit. Care Med. 2010, 181, 315–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siroux, V.; Oryszczyn, M.P.; Paty, E.; Kauffmann, F.; Pison, C.; Vervloet, D.; Pin, I. Relationships of allergic sensitization, total immunoglobulin E and blood eosinophils to asthma severity in children of the EGEA Study. Clin. Exp. Allergy 2003, 33, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, A.M.; Gaston, B.M.; Erzurum, S.C.; Teague, W.G. Features of severe asthma in school-age children: Atopy and increased exhaled nitric oxide. J. Allergy Clin. Immunol. 2006, 118, 1218–1225. [Google Scholar] [CrossRef] [Green Version]
- Holt, P.G.; Upham, J.W.; Sly, P.D. Contemporaneous maturation of immunologic and respiratory functions during early childhood: Implications for development of asthma prevention strategies. J. Allergy Clin. Immunol. 2005, 116, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Bui, D.S.; Lodge, C.J.; Perret, J.L.; Lowe, A.; Hamilton, G.S.; Thompson, B.; Giles, G.; Tan, D.; Erbas, B.; Pirkis, J. Trajectories of asthma and allergies from 7 years to 53 years and associations with lung function and extrapulmonary comorbidity profiles: A prospective cohort study. Lancet Respir. Med. 2021, 9, 387–396. [Google Scholar] [CrossRef]
- To, M.; Tsuzuki, R.; Katsube, O.; Yamawaki, S.; Soeda, S.; Kono, Y.; Honda, N.; Kano, I.; Haruki, K.; To, Y. Persistent Asthma from Childhood to Adulthood Presents a Distinct Phenotype of Adult Asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 1921–1927.e2. [Google Scholar] [CrossRef]
- Sears, M.R.; Greene, J.M.; Willan, A.R.; Wiecek, E.M.; Taylor, D.R.; Flannery, E.M.; Cowan, J.O.; Herbison, G.P.; Silva, P.A.; Poulton, R. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N. Engl. J. Med. 2003, 349, 1414–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maruo, H.; Hashimoto, K.; Shimoda, K.; Shimanuki, K.; Nakayama, T.; Yamaguchi, H.; Shiigai, N.; Uchimura, K.; Mitsubayashi, T.; Akasaka, T. Long-term follow-up studies of bronchial asthma in children. I. Prognosis and risk factors. Arerugi=[Allergy] 1990, 39, 621–630. [Google Scholar] [PubMed]
- Andersson, M.; Hedman, L.; Bjerg, A.; Forsberg, B.; Lundbäck, B.; Rönmark, E. Remission and persistence of asthma followed from 7 to 19 years of age. Pediatrics 2013, 132, e435–e442. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.E. Complex phenotypes in asthma: Current definitions. Pulm. Pharmacol. Ther. 2013, 26, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Agache, I.; Akdis, C.; Jutel, M.; Virchow, J.C. Untangling asthma phenotypes and endotypes. Allergy 2012, 67, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.E. Asthma: Defining of the persistent adult phenotypes. Lancet 2006, 368, 804–813. [Google Scholar] [CrossRef]
- Lötvall, J.; Akdis, C.A.; Bacharier, L.B.; Bjermer, L.; Casale, T.B.; Custovic, A.; Lemanske, R.F., Jr.; Wardlaw, A.J.; Wenzel, S.E.; Greenberger, P.A. Asthma endotypes: A new approach to classification of disease entities within the asthma syndrome. J. Allergy Clin. Immunol. 2011, 127, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Anto, J.M.; Wickman, M.; Keil, T.; Valenta, R.; Haahtela, T.; Lodrup Carlsen, K.; van Hage, M.; Akdis, C.; Bachert, C.; et al. Are allergic multimorbidities and IgE polysensitization associated with the persistence or re-occurrence of foetal type 2 signalling? The MeDALL hypothesis. Allergy 2015, 70, 1062–1078. [Google Scholar] [CrossRef] [PubMed]
- Pongracic, J.A.; Krouse, R.Z.; Babineau, D.C.; Zoratti, E.M.; Cohen, R.T.; Wood, R.A.; Khurana Hershey, G.K.; Kercsmar, C.M.; Gruchalla, R.S.; Kattan, M.; et al. Distinguishing characteristics of difficult-to-control asthma in inner-city children and adolescents. J. Allergy Clin. Immunol. 2016, 138, 1030–1041. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.H.; Babineau, D.C.; Krouse, R.Z.; Zoratti, E.M.; Pongracic, J.A.; O’Connor, G.T.; Wood, R.A.; Khurana Hershey, G.K.; Kercsmar, C.M.; Gruchalla, R.S.; et al. Pathways through which asthma risk factors contribute to asthma severity in inner-city children. J. Allergy Clin. Immunol. 2016, 138, 1042–1050. [Google Scholar] [CrossRef] [Green Version]
- Dufrois, C.; Bourgoin-Heck, M.; Lambert, N.; Just, J.; Bregeon, A.; Taillé, C.; Wanin, S. Maintenance of Asthma Control in Adolescents with Severe Asthma After Transitioning to a Specialist Adult Centre: A French Cohort Experience. J. Asthma Allergy 2022, 15, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Wechsler, M.E.; Tran, T.N.; Heaney, L.G.; Jones, R.C.; Menzies-Gow, A.N.; Busby, J.; Jackson, D.J.; Pfeffer, P.E.; Rhee, C.K.; et al. Characterization of Severe Asthma Worldwide: Data from the International Severe Asthma Registry. Chest 2020, 157, 790–804. [Google Scholar] [CrossRef] [PubMed]
- Louhaichi, S.; Hamdi, B.; Khalfallah, I.; Akkad, A.; Ammar, J.; Berraies, A.; Hamzaoui, A. Analysis of phenotypes in a group of severe asthma. Eur. Respir. J. 2019, 54 (Suppl. S63), PA4098. [Google Scholar] [CrossRef]
- Illi, S.; von Mutius, E.; Lau, S.; Niggemann, B.; Grüber, C.; Wahn, U. Perennial allergen sensitisation early in life and chronic asthma in children: A birth cohort study. Lancet 2006, 368, 763–770. [Google Scholar] [CrossRef]
- Jartti, T.; Gern, J.E. Role of viral infections in the development and exacerbation of asthma in children. J. Allergy Clin. Immunol. 2017, 140, 895–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadopoulos, N.G.; Christodoulou, I.; Rohde, G.; Agache, I.; Almqvist, C.; Bruno, A.; Bonini, S.; Bont, L.; Bossios, A.; Bousquet, J.; et al. Viruses and bacteria in acute asthma exacerbations—A GA2 LEN-DARE systematic review. Allergy 2011, 66, 458–468. [Google Scholar] [CrossRef]
- Custovic, A. To what extent is allergen exposure a risk factor for the development of allergic disease? Clin. Exp. Allergy 2015, 45, 54–62. [Google Scholar] [CrossRef]
- Xepapadaki, P.; Bachert, C.; Finotto, S.; Jartti, T.; Konstantinou, G.N.; Kiefer, A.; Kowalski, M.; Lewandowska-Polak, A.; Lukkarinen, H.; Roumpedaki, E.; et al. Contribution of repeated infections in asthma persistence from preschool to school age: Design and characteristics of the PreDicta cohort. Pediatr. Allergy Immunol. 2018, 29, 383–393. [Google Scholar] [CrossRef]
- Lefaudeux, D.; De Meulder, B.; Loza, M.J.; Peffer, N.; Rowe, A.; Baribaud, F.; Bansal, A.T.; Lutter, R.; Sousa, A.R.; Corfield, J.; et al. U-BIOPRED clinical adult asthma clusters linked to a subset of sputum omics. J. Allergy Clin. Immunol. 2017, 139, 1797–1807. [Google Scholar] [CrossRef] [Green Version]
- Haldar, P.; Pavord, I.D.; Shaw, D.E.; Berry, M.A.; Thomas, M.; Brightling, C.E.; Wardlaw, A.J.; Green, R.H. Cluster analysis and clinical asthma phenotypes. Am. J. Respir. Crit. Care Med. 2008, 178, 218–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, R.; Chupp, G. Phenotypes and endotypes of adult asthma: Moving toward precision medicine. J. Allergy Clin. Immunol. 2019, 144, 1–12. [Google Scholar] [CrossRef]
- Fahy, J.V. Type 2 inflammation in asthma--present in most, absent in many. Nat. Rev. Immunol. 2015, 15, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, M.E.; Lee, F.E.; Lee, G.B. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Akdis, C.A. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat. Rev. Immunol. 2021, 21, 739–751. [Google Scholar] [CrossRef]
- Mitchell, P.D.; O’Byrne, P.M. Epithelial-Derived Cytokines in Asthma. Chest 2017, 151, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Gause, W.C.; Wynn, T.A.; Allen, J.E. Type 2 immunity and wound healing: Evolutionary refinement of adaptive immunity by helminths. Nat. Rev. Immunol. 2013, 13, 607–614. [Google Scholar] [CrossRef] [Green Version]
- Holgate, S.T. Innate and adaptive immune responses in asthma. Nat. Med. 2012, 18, 673–683. [Google Scholar] [CrossRef]
- Morgan, L.E.; Jaramillo, A.M.; Shenoy, S.K.; Raclawska, D.; Emezienna, N.A.; Richardson, V.L.; Hara, N.; Harder, A.Q.; NeeDell, J.C.; Hennessy, C.E.; et al. Disulfide disruption reverses mucus dysfunction in allergic airway disease. Nat. Commun. 2021, 12, 249. [Google Scholar] [CrossRef]
- Fahy, J.V.; Dickey, B.F. Airway mucus function and dysfunction. N. Engl. J. Med. 2010, 363, 2233–2247. [Google Scholar] [CrossRef] [Green Version]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef] [Green Version]
- Pavord, I.D.; Beasley, R.; Agusti, A.; Anderson, G.P.; Bel, E.; Brusselle, G.; Cullinan, P.; Custovic, A.; Ducharme, F.M.; Fahy, J.V.; et al. After asthma: Redefining airways diseases. Lancet 2018, 391, 350–400. [Google Scholar] [CrossRef]
- Akdis, M.; Aab, A.; Altunbulakli, C.; Azkur, K.; Costa, R.A.; Crameri, R.; Duan, S.; Eiwegger, T.; Eljaszewicz, A.; Ferstl, R.; et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: Receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 2016, 138, 984–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, H.F.; Phipps, S.; Foster, P.S. Eosinophil trafficking in allergy and asthma. J. Allergy Clin. Immunol. 2007, 119, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Chanez, P.; Lacoste, J.Y.; Barnéon, G.; Ghavanian, N.; Enander, I.; Venge, P.; Ahlstedt, S.; Simony-Lafontaine, J.; Godard, P.; et al. Eosinophilic inflammation in asthma. N. Engl. J. Med. 1990, 323, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate Lymphoid Cells: 10 Years On. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peebles, R.S., Jr.; Aronica, M.A. Proinflammatory Pathways in the Pathogenesis of Asthma. Clin. Chest Med. 2019, 40, 29–50. [Google Scholar] [CrossRef] [PubMed]
- Brusselle, G.G.; Maes, T.; Bracke, K.R. Eosinophils in the spotlight: Eosinophilic airway inflammation in nonallergic asthma. Nat. Med. 2013, 19, 977–979. [Google Scholar] [CrossRef]
- Moffatt, M.F.; Gut, I.G.; Demenais, F.; Strachan, D.P.; Bouzigon, E.; Heath, S.; von Mutius, E.; Farrall, M.; Lathrop, M.; Cookson, W.O. A large-scale, consortium-based genomewide association study of asthma. N. Engl. J. Med. 2010, 363, 1211–1221. [Google Scholar] [CrossRef] [Green Version]
- Barnig, C.; Cernadas, M.; Dutile, S.; Liu, X.; Perrella, M.A.; Kazani, S.; Wechsler, M.E.; Israel, E.; Levy, B.D. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci. Transl. Med. 2013, 5, 174ra26. [Google Scholar] [CrossRef] [Green Version]
- Oppenheimer, J.; Hoyte, F.C.; Phipatanakul, W.; Silver, J.; Howarth, P.; Lugogo, N.L. Allergic and eosinophilic asthma in the era of biomarkers and biologics: Similarities, differences and misconceptions. Ann. Allergy Asthma Immunol. 2022; in press. [Google Scholar] [CrossRef]
- Psarras, S.; Volonaki, E.; Skevaki, C.L.; Xatzipsalti, M.; Bossios, A.; Pratsinis, H.; Tsigkos, S.; Gourgiotis, D.; Constantopoulos, A.G.; Papapetropoulos, A.; et al. Vascular endothelial growth factor-mediated induction of angiogenesis by human rhinoviruses. J. Allergy Clin. Immunol. 2006, 117, 291–297. [Google Scholar] [CrossRef]
- Skevaki, C.L.; Psarras, S.; Volonaki, E.; Pratsinis, H.; Spyridaki, I.S.; Gaga, M.; Georgiou, V.; Vittorakis, S.; Telcian, A.G.; Maggina, P.; et al. Rhinovirus-induced basic fibroblast growth factor release mediates airway remodeling features. Clin. Transl. Allergy 2012, 2, 14. [Google Scholar] [CrossRef] [Green Version]
- Xepapadaki, P.; Papadopoulos, N.G.; Bossios, A.; Manoussakis, E.; Manousakas, T.; Saxoni-Papageorgiou, P. Duration of postviral airway hyperresponsiveness in children with asthma: Effect of atopy. J. Allergy Clin. Immunol. 2005, 116, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Oddy, W.H.; de Klerk, N.H.; Sly, P.D.; Holt, P.G. The effects of respiratory infections, atopy, and breastfeeding on childhood asthma. Eur. Respir. J. 2002, 19, 899–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Diamant, Z.; Vijverberg, S.; Alving, K.; Bakirtas, A.; Bjermer, L.; Custovic, A.; Dahlen, S.E.; Gaga, M.; Gerth van Wijk, R.; Giacco, S.D.; et al. Toward clinically applicable biomarkers for asthma: An EAACI position paper. Allergy 2019, 74, 1835–1851. [Google Scholar] [CrossRef] [Green Version]
- Brusselle, G.G.; Koppelman, G.H. Biologic Therapies for Severe Asthma. N. Engl. J. Med. 2022, 386, 157–171. [Google Scholar] [CrossRef]
- Ballardini, N.; Bergström, A.; Wahlgren, C.F.; van Hage, M.; Hallner, E.; Kull, I.; Melén, E.; Antó, J.M.; Bousquet, J.; Wickman, M. IgE antibodies in relation to prevalence and multimorbidity of eczema, asthma, and rhinitis from birth to adolescence. Allergy 2016, 71, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.; Ollert, M.; Aalberse, R.; Austin, M.; Custovic, A.; DunnGalvin, A.; Eigenmann, P.A.; Fassio, F.; Grattan, C.; Hellings, P.; et al. A new framework for the interpretation of IgE sensitization tests. Allergy 2016, 71, 1540–1551. [Google Scholar] [CrossRef] [Green Version]
- Fontanella, S.; Frainay, C.; Murray, C.S.; Simpson, A.; Custovic, A. Machine learning to identify pairwise interactions between specific IgE antibodies and their association with asthma: A cross-sectional analysis within a population-based birth cohort. PLoS Med. 2018, 15, e1002691. [Google Scholar] [CrossRef]
- Holt, P.G.; Strickland, D.; Bosco, A.; Belgrave, D.; Hales, B.; Simpson, A.; Hollams, E.; Holt, B.; Kusel, M.; Ahlstedt, S.; et al. Distinguishing benign from pathologic TH2 immunity in atopic children. J. Allergy Clin. Immunol. 2016, 137, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Dweik, R.A.; Sorkness, R.L.; Wenzel, S.; Hammel, J.; Curran-Everett, D.; Comhair, S.A.; Bleecker, E.; Busse, W.; Calhoun, W.J.; Castro, M.; et al. Use of exhaled nitric oxide measurement to identify a reactive, at-risk phenotype among patients with asthma. Am. J. Respir. Crit. Care Med. 2010, 181, 1033–1041. [Google Scholar] [CrossRef] [Green Version]
- ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am. J. Respir. Crit. Care Med. 2005, 171, 912–930. [CrossRef] [PubMed] [Green Version]
- Xepapadaki, P.; Korovessi, P.; Bachert, C.; Finotto, S.; Jartti, T.; Lakoumentas, J.; Kowalski, M.L.; Lewandowska-Polak, A.; Lukkarinen, H.; Zhang, N.; et al. Evolution of Airway Inflammation in Preschoolers with Asthma-Results of a Two-Year Longitudinal Study. J. Clin. Med. 2020, 9, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korevaar, D.A.; Westerhof, G.A.; Wang, J.; Cohen, J.F.; Spijker, R.; Sterk, P.J.; Bel, E.H.; Bossuyt, P.M. Diagnostic accuracy of minimally invasive markers for detection of airway eosinophilia in asthma: A systematic review and meta-analysis. Lancet Respir. Med. 2015, 3, 290–300. [Google Scholar] [CrossRef]
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Irvin, C.G.; Leigh, M.W.; Lundberg, J.O.; Olin, A.C.; Plummer, A.L.; Taylor, D.R. An official ATS clinical practice guideline: Interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am. J. Respir. Crit. Care Med. 2011, 184, 602–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karrasch, S.; Linde, K.; Rücker, G.; Sommer, H.; Karsch-Völk, M.; Kleijnen, J.; Jörres, R.A.; Schneider, A. Accuracy of FENO for diagnosing asthma: A systematic review. Thorax 2017, 72, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Woodruff, P.G.; Modrek, B.; Choy, D.F.; Jia, G.; Abbas, A.R.; Ellwanger, A.; Koth, L.L.; Arron, J.R.; Fahy, J.V. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am. J. Respir. Crit. Care Med. 2009, 180, 388–395. [Google Scholar] [CrossRef] [Green Version]
- Gibson, P.G. Inflammatory phenotypes in adult asthma: Clinical applications. Clin. Respir. J. 2009, 3, 198–206. [Google Scholar] [CrossRef]
- Petsky, H.L.; Cates, C.J.; Kew, K.M.; Chang, A.B. Tailoring asthma treatment on eosinophilic markers (exhaled nitric oxide or sputum eosinophils): A systematic review and meta-analysis. Thorax 2018, 73, 1110–1119. [Google Scholar] [CrossRef] [Green Version]
- Global Initiative for Asthma. 2022 GINA Report, Global Management for Asthma Management and Prevention (2022 Update). Available online: https://ginasthma.org/gina-reports (accessed on 4 June 2022).
- Holguin, F.; Cardet, J.C.; Chung, K.F.; Diver, S.; Ferreira, D.S.; Fitzpatrick, A.; Gaga, M.; Kellermeyer, L.; Khurana, S.; Knight, S.; et al. Management of severe asthma: A European Respiratory Society/American Thoracic Society guideline. Eur. Respir. J. 2020, 55, 1900588. [Google Scholar] [CrossRef] [Green Version]
- Ullmann, N.; Bossley, C.J.; Fleming, L.; Silvestri, M.; Bush, A.; Saglani, S. Blood eosinophil counts rarely reflect airway eosinophilia in children with severe asthma. Allergy 2013, 68, 402–406. [Google Scholar] [CrossRef]
- Kumar, R.M.; Pajanivel, R.; Koteeswaran, G.; Menon, S.K.; Charles, P.M. Correlation of total serum immunoglobulin E level, sputum, and peripheral eosinophil count in assessing the clinical severity in bronchial asthma. Lung India 2017, 34, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Zoratti, E.; Havstad, S.; Wegienka, G.; Nicholas, C.; Bobbitt, K.R.; Woodcroft, K.J.; Ownby, D.R.; Johnson, C.C. Differentiating asthma phenotypes in young adults through polyclonal cytokine profiles. Ann. Allergy Asthma Immunol. 2014, 113, 25–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humbert, M.; Taillé, C.; Mala, L.; Le Gros, V.; Just, J.; Molimard, M. Omalizumab effectiveness in patients with severe allergic asthma according to blood eosinophil count: The STELLAIR study. Eur. Respir. J. 2018, 51, 1702523. [Google Scholar] [CrossRef] [PubMed]
- Casale, T.B.; Luskin, A.T.; Busse, W.; Zeiger, R.S.; Trzaskoma, B.; Yang, M.; Griffin, N.M.; Chipps, B.E. Omalizumab Effectiveness by Biomarker Status in Patients with Asthma: Evidence from PROSPERO, A Prospective Real-World Study. J. Allergy Clin. Immunol. Pract. 2019, 7, 156–164.e1. [Google Scholar] [CrossRef]
- Hanania, N.A.; Wenzel, S.; Rosén, K.; Hsieh, H.J.; Mosesova, S.; Choy, D.F.; Lal, P.; Arron, J.R.; Harris, J.M.; Busse, W. Exploring the effects of omalizumab in allergic asthma: An analysis of biomarkers in the EXTRA study. Am. J. Respir. Crit. Care Med. 2013, 187, 804–811. [Google Scholar] [CrossRef]
- Izuhara, K.; Conway, S.J.; Moore, B.B.; Matsumoto, H.; Holweg, C.T.; Matthews, J.G.; Arron, J.R. Roles of Periostin in Respiratory Disorders. Am. J. Respir. Crit. Care Med. 2016, 193, 949–956. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Meguro, K.; Kawashima, H.; Kashiwakuma, D.; Kagami, S.I.; Ohta, S.; Ono, J.; Izuhara, K.; Iwamoto, I. Serum periostin levels serve as a biomarker for both eosinophilic airway inflammation and fixed airflow limitation in well-controlled asthmatics. J. Asthma 2019, 56, 236–243. [Google Scholar] [CrossRef]
- Matsumoto, H. Role of serum periostin in the management of asthma and its comorbidities. Respir. Investig. 2020, 58, 144–154. [Google Scholar] [CrossRef]
- Noguchi, T.; Nakagome, K.; Kobayashi, T.; Uchida, Y.; Soma, T.; Nakamoto, H.; Nagata, M. Periostin upregulates the effector functions of eosinophils. J. Allergy Clin. Immunol. 2016, 138, 1449–1452.e5. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, H. Serum periostin: A novel biomarker for asthma management. Allergol. Int. 2014, 63, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Chiappori, A.; De Ferrari, L.; Folli, C.; Mauri, P.; Riccio, A.M.; Canonica, G.W. Biomarkers and severe asthma: A critical appraisal. Clin. Mol. Allergy 2015, 13, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, A.; Hedlin, G. Biomarkers for the Phenotyping and Monitoring of Asthma in Children. Curr. Treat. Options Allergy 2016, 3, 439–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabito, F.A.; Carlson, J.C.; He, H.; Werthmann, D.; Schal, C. A single intervention for cockroach control reduces cockroach exposure and asthma morbidity in children. J. Allergy Clin. Immunol. 2017, 140, 565–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crocker, D.D.; Kinyota, S.; Dumitru, G.G.; Ligon, C.B.; Herman, E.J.; Ferdinands, J.M.; Hopkins, D.P.; Lawrence, B.M.; Sipe, T.A. Effectiveness of home-based, multi-trigger, multicomponent interventions with an environmental focus for reducing asthma morbidity: A community guide systematic review. Am. J. Prev. Med. 2011, 41 (Suppl. S1), S5–S32. [Google Scholar] [CrossRef] [PubMed]
- Morgan, W.J.; Crain, E.F.; Gruchalla, R.S.; O’Connor, G.T.; Kattan, M.; Evans, R., 3rd; Stout, J.; Malindzak, G.; Smartt, E.; Plaut, M.; et al. Results of a home-based environmental intervention among urban children with asthma. N. Engl. J. Med. 2004, 351, 1068–1080. [Google Scholar] [CrossRef] [Green Version]
- Murray, C.S.; Foden, P.; Sumner, H.; Shepley, E.; Custovic, A.; Simpson, A. Preventing Severe Asthma Exacerbations in Children. A Randomized Trial of Mite-Impermeable Bedcovers. Am. J. Respir. Crit. Care Med. 2017, 196, 150–158. [Google Scholar] [CrossRef]
- Barnes, P.J. Inhaled corticosteroids. Pharmaceuticals 2010, 3, 514–540. [Google Scholar] [CrossRef] [Green Version]
- Deykin, A.; Lazarus, S.C.; Fahy, J.V.; Wechsler, M.E.; Boushey, H.A.; Chinchilli, V.M.; Craig, T.J.; Dimango, E.; Kraft, M.; Leone, F. Sputum eosinophil counts predict asthma control after discontinuation of inhaled corticosteroids. J. Allergy Clin. Immunol. 2005, 115, 720–727. [Google Scholar] [CrossRef]
- Szefler, S.J.; Phillips, B.R.; Martinez, F.D.; Chinchilli, V.M.; Lemanske, R.F.; Strunk, R.C.; Zeiger, R.S.; Larsen, G.; Spahn, J.D.; Bacharier, L.B.; et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J. Allergy Clin. Immunol. 2005, 115, 233–242. [Google Scholar] [CrossRef]
- Bacharier, L.B.; Guilbert, T.W.; Zeiger, R.S.; Strunk, R.C.; Morgan, W.J.; Lemanske, R.F., Jr.; Moss, M.; Szefler, S.J.; Krawiec, M.; Boehmer, S.; et al. Patient characteristics associated with improved outcomes with use of an inhaled corticosteroid in preschool children at risk for asthma. J. Allergy Clin. Immunol. 2009, 123, 1077–1082.e5. [Google Scholar] [CrossRef] [Green Version]
- Fitzpatrick, A.M.; Jackson, D.J.; Mauger, D.T.; Boehmer, S.J.; Phipatanakul, W.; Sheehan, W.J.; Moy, J.N.; Paul, I.M.; Bacharier, L.B.; Cabana, M.D.; et al. Individualized therapy for persistent asthma in young children. J. Allergy Clin. Immunol. 2016, 138, 1608–1618.e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, J.A.; Lazarus, S.C.; Blake, K.V.; Sorkness, C.A.; Covar, R.; Dyer, A.M.; Lang, J.E.; Lugogo, N.L.; Mauger, D.T.; Wechsler, M.E.; et al. Biomarkers to Predict Response to Inhaled Corticosteroids and Long-Acting Muscarinic Antagonists in Adolescents and Adults with Mild Persistent Asthma. Ann. Am. Thorac. Soc. 2022, 19, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Knuffman, J.E.; Sorkness, C.A.; Lemanske, R.F., Jr.; Mauger, D.T.; Boehmer, S.J.; Martinez, F.D.; Bacharier, L.B.; Strunk, R.C.; Szefler, S.J.; Zeiger, R.S.; et al. Phenotypic predictors of long-term response to inhaled corticosteroid and leukotriene modifier therapies in pediatric asthma. J. Allergy Clin. Immunol. 2009, 123, 411–416. [Google Scholar] [CrossRef] [Green Version]
- Marcon, A.; Marchetti, P.; Antó, J.M.; Cazzoletti, L.; Cerveri, I.; Corsico, A.; Ferreira, D.S.; Garcia-Aymerich, J.; Gislason, D.; Heinrich, J.; et al. Atopy Modifies the Association Between Inhaled Corticosteroid Use and Lung Function Decline in Patients with Asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 980–988.e10. [Google Scholar] [CrossRef] [PubMed]
- Mitsias, D.I.; Xepapadaki, P.; Makris, M.; Papadopoulos, N.G. Immunotherapy in allergic diseases—Improved understanding and innovation for enhanced effectiveness. Curr. Opin. Immunol. 2020, 66, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Halken, S.; Larenas-Linnemann, D.; Roberts, G.; Calderón, M.A.; Angier, E.; Pfaar, O.; Ryan, D.; Agache, I.; Ansotegui, I.J.; Arasi, S.; et al. EAACI guidelines on allergen immunotherapy: Prevention of allergy. Pediatr. Allergy Immunol. 2017, 28, 728–745. [Google Scholar] [CrossRef]
- Pitsios, C.; Demoly, P.; Bilò, M.B.; Gerth van Wijk, R.; Pfaar, O.; Sturm, G.J.; Rodriguez del Rio, P.; Tsoumani, M.; Gawlik, R.; Paraskevopoulos, G.; et al. Clinical contraindications to allergen immunotherapy: An EAACI position paper. Allergy 2015, 70, 897–909. [Google Scholar] [CrossRef]
- Pajno, G.B.; Barberio, G.; De Luca, F.; Morabito, L.; Parmiani, S. Prevention of new sensitizations in asthmatic children monosensitized to house dust mite by specific immunotherapy. A six-year follow-up study. Clin. Exp. Allergy 2001, 31, 1392–1397. [Google Scholar] [CrossRef]
- Tosca, M.A.; Licari, A.; Olcese, R.; Marseglia, G.; Sacco, O.; Ciprandi, G. Immunotherapy and Asthma in Children. Front. Pediatr. 2018, 6, 231. [Google Scholar] [CrossRef]
- Agache, I.; Lau, S.; Akdis, C.A.; Smolinska, S.; Bonini, M.; Cavkaytar, O.; Flood, B.; Gajdanowicz, P.; Izuhara, K.; Kalayci, O.; et al. EAACI Guidelines on Allergen Immunotherapy: House dust mite-driven allergic asthma. Allergy 2019, 74, 855–873. [Google Scholar] [CrossRef] [Green Version]
- Brożek, J.L.; Bousquet, J.; Agache, I.; Agarwal, A.; Bachert, C.; Bosnic-Anticevich, S.; Brignardello-Petersen, R.; Canonica, G.W.; Casale, T.; Chavannes, N.H.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J. Allergy Clin. Immunol. 2017, 140, 950–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jutel, M.; Agache, I.; Bonini, S.; Burks, A.W.; Calderon, M.; Canonica, W.; Cox, L.; Demoly, P.; Frew, A.J.; O’Hehir, R.; et al. International consensus on allergy immunotherapy. J. Allergy Clin. Immunol. 2015, 136, 556–568. [Google Scholar] [CrossRef] [Green Version]
- Dhami, S.; Kakourou, A.; Asamoah, F.; Agache, I.; Lau, S.; Jutel, M.; Muraro, A.; Roberts, G.; Akdis, C.A.; Bonini, M.; et al. Allergen immunotherapy for allergic asthma: A systematic review and meta-analysis. Allergy 2017, 72, 1825–1848. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, N.G.; Arakawa, H.; Carlsen, K.H.; Custovic, A.; Gern, J.; Lemanske, R.; Le Souef, P.; Mäkelä, M.; Roberts, G.; Wong, G.; et al. International consensus on (ICON) pediatric asthma. Allergy 2012, 67, 976–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abramson, M.J.; Puy, R.M.; Weiner, J.M. Allergen immunotherapy for asthma. Cochrane Database Syst. Rev. 2003, 4, CD001186. [Google Scholar] [CrossRef]
- Abrams, E.M.; Szefler, S.J.; Becker, A.B. Effect of asthma therapies on the natural course of asthma. Ann. Allergy Asthma Immunol. 2016, 117, 627–633. [Google Scholar] [CrossRef] [Green Version]
- Cox, L.; Nelson, H.; Lockey, R.; Calabria, C.; Chacko, T.; Finegold, I.; Nelson, M.; Weber, R.; Bernstein, D.I.; Blessing-Moore, J.; et al. Allergen immunotherapy: A practice parameter third update. J. Allergy Clin. Immunol. 2011, 127 (Suppl. S1), S1–S55. [Google Scholar] [CrossRef]
- Har, D.; Lee, M.J. Systemic reaction rates with omalizumab, subcutaneous immunotherapy, and combination therapy in children with allergic asthma. Allergy Asthma Proc. 2019, 40, 35–40. [Google Scholar] [CrossRef]
- Holgate, S.; Casale, T.; Wenzel, S.; Bousquet, J.; Deniz, Y.; Reisner, C. The anti-inflammatory effects of omalizumab confirm the central role of IgE in allergic inflammation. J. Allergy Clin. Immunol. 2005, 115, 459–465. [Google Scholar] [CrossRef]
- Brusselle, G.; Michils, A.; Louis, R.; Dupont, L.; Van de Maele, B.; Delobbe, A.; Pilette, C.; Lee, C.S.; Gurdain, S.; Vancayzeele, S.; et al. “Real-life” effectiveness of omalizumab in patients with severe persistent allergic asthma: The PERSIST study. Respir. Med. 2009, 103, 1633–1642. [Google Scholar] [CrossRef]
- Busse, W.; Corren, J.; Lanier, B.Q.; McAlary, M.; Fowler-Taylor, A.; Cioppa, G.D.; van As, A.; Gupta, N. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J. Allergy Clin. Immunol. 2001, 108, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Busse, W.W.; Morgan, W.J.; Gergen, P.J.; Mitchell, H.E.; Gern, J.E.; Liu, A.H.; Gruchalla, R.S.; Kattan, M.; Teach, S.J.; Pongracic, J.A.; et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N. Engl. J. Med. 2011, 364, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Normansell, R.; Walker, S.; Milan, S.J.; Walters, E.H.; Nair, P. Omalizumab for asthma in adults and children. Cochrane Database Syst. Rev. 2014, 1, CD003559. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; Corren, J.; Pavord, I.D.; Maspero, J.; Wenzel, S.; Rabe, K.F.; Busse, W.W.; Ford, L.; Sher, L.; FitzGerald, J.M.; et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N. Engl. J. Med. 2018, 378, 2486–2496. [Google Scholar] [CrossRef]
- Castro, M.; Rabe, K.F.; Corren, J.; Pavord, I.D.; Katelaris, C.H.; Tohda, Y.; Zhang, B.; Rice, M.S.; Maroni, J.; Rowe, P.; et al. Dupilumab improves lung function in patients with uncontrolled, moderate-to-severe asthma. ERJ Open Res. 2020, 6, 00204-2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabe, K.F.; Nair, P.; Brusselle, G.; Maspero, J.F.; Castro, M.; Sher, L.; Zhu, H.; Hamilton, J.D.; Swanson, B.N.; Khan, A.; et al. Efficacy and Safety of Dupilumab in Glucocorticoid-Dependent Severe Asthma. N. Engl. J. Med. 2018, 378, 2475–2485. [Google Scholar] [CrossRef]
- Menzies-Gow, A.; Mansur, A.H.; Brightling, C.E. Clinical utility of fractional exhaled nitric oxide in severe asthma management. Eur. Respir. J. 2020, 55, 1901633. [Google Scholar] [CrossRef]
- Bachert, C.; Han, J.K.; Desrosiers, M.; Hellings, P.W.; Amin, N.; Lee, S.E.; Mullol, J.; Greos, L.S.; Bosso, J.V.; Laidlaw, T.M.; et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): Results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 2019, 394, 1638–1650. [Google Scholar] [CrossRef] [Green Version]
- Thaçi, D.; Simpson, E.L.; Beck, L.A.; Bieber, T.; Blauvelt, A.; Papp, K.; Soong, W.; Worm, M.; Szepietowski, J.C.; Sofen, H.; et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: A randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet 2016, 387, 40–52. [Google Scholar] [CrossRef]
- Dupin, C.; Belhadi, D.; Guilleminault, L.; Gamez, A.S.; Berger, P.; De Blay, F.; Bonniaud, P.; Leroyer, C.; Mahay, G.; Girodet, P.O.; et al. Effectiveness and safety of dupilumab for the treatment of severe asthma in a real-life French multi-centre adult cohort. Clin. Exp. Allergy 2020, 50, 789–798. [Google Scholar] [CrossRef]
- Pavord, I.D.; Korn, S.; Howarth, P.; Bleecker, E.R.; Buhl, R.; Keene, O.N.; Ortega, H.; Chanez, P. Mepolizumab for severe eosinophilic asthma (DREAM): A multicentre, double-blind, placebo-controlled trial. Lancet 2012, 380, 651–659. [Google Scholar] [CrossRef]
- Ortega, H.G.; Liu, M.C.; Pavord, I.D.; Brusselle, G.G.; FitzGerald, J.M.; Chetta, A.; Humbert, M.; Katz, L.E.; Keene, O.N.; Yancey, S.W.; et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N. Engl. J. Med. 2014, 371, 1198–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bel, E.H.; Wenzel, S.E.; Thompson, P.J.; Prazma, C.M.; Keene, O.N.; Yancey, S.W.; Ortega, H.G.; Pavord, I.D. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N. Engl. J. Med. 2014, 371, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; Zangrilli, J.; Wechsler, M.E.; Bateman, E.D.; Brusselle, G.G.; Bardin, P.; Murphy, K.; Maspero, J.F.; O’Brien, C.; Korn, S. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: Results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir. Med. 2015, 3, 355–366. [Google Scholar] [CrossRef]
- Bjermer, L.; Lemiere, C.; Maspero, J.; Weiss, S.; Zangrilli, J.; Germinaro, M. Reslizumab for Inadequately Controlled Asthma With Elevated Blood Eosinophil Levels: A Randomized Phase 3 Study. Chest 2016, 150, 789–798. [Google Scholar] [CrossRef] [Green Version]
- Bleecker, E.R.; FitzGerald, J.M.; Chanez, P.; Papi, A.; Weinstein, S.F.; Barker, P.; Sproule, S.; Gilmartin, G.; Aurivillius, M.; Werkström, V.; et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β(2)-agonists (SIROCCO): A randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016, 388, 2115–2127. [Google Scholar] [CrossRef]
- FitzGerald, J.M.; Bleecker, E.R.; Nair, P.; Korn, S.; Ohta, K.; Lommatzsch, M.; Ferguson, G.T.; Busse, W.W.; Barker, P.; Sproule, S.; et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016, 388, 2128–2141. [Google Scholar] [CrossRef]
- Nair, P.; Wenzel, S.; Rabe, K.F.; Bourdin, A.; Lugogo, N.L.; Kuna, P.; Barker, P.; Sproule, S.; Ponnarambil, S.; Goldman, M. Oral Glucocorticoid-Sparing Effect of Benralizumab in Severe Asthma. N. Engl. J. Med. 2017, 376, 2448–2458. [Google Scholar] [CrossRef]

| Key Points |
|---|
| Allergic asthma is the most common phenotype of asthma. It is associated with sensitization to environmental allergens and asthma related symptoms upon exposure. |
| A T2 inflammatory pathway contributes to allergic asthma mechanisms. |
| Allergic comorbidities such as allergic rhinitis and atopic dermatitis often coexist with allergic asthma. |
| Diagnosis of allergic asthma is based on in vivo (skin prick tests) and/or in vitro (allergen specific IgE levels, Component Resolved Diagnosis) documentation of allergic sensitization. |
| Specific biomarkers such as serum IgE, eosinophils, and epidemiological characteristics (early-onset, atopic comorbidities) contribute to identifying patients with allergic asthma. |
| Besides inhaled treatments, allergen avoidance, specific immunotherapy, and biologicals represent additional therapeutic options for allergic asthma. |
| Biologics targeting T2 inflammation exist for management of allergic eosinophilic asthma. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papapostolou, N.; Makris, M. Allergic Asthma in the Era of Personalized Medicine. J. Pers. Med. 2022, 12, 1162. https://doi.org/10.3390/jpm12071162
Papapostolou N, Makris M. Allergic Asthma in the Era of Personalized Medicine. Journal of Personalized Medicine. 2022; 12(7):1162. https://doi.org/10.3390/jpm12071162
Chicago/Turabian StylePapapostolou, Niki, and Michael Makris. 2022. "Allergic Asthma in the Era of Personalized Medicine" Journal of Personalized Medicine 12, no. 7: 1162. https://doi.org/10.3390/jpm12071162
APA StylePapapostolou, N., & Makris, M. (2022). Allergic Asthma in the Era of Personalized Medicine. Journal of Personalized Medicine, 12(7), 1162. https://doi.org/10.3390/jpm12071162