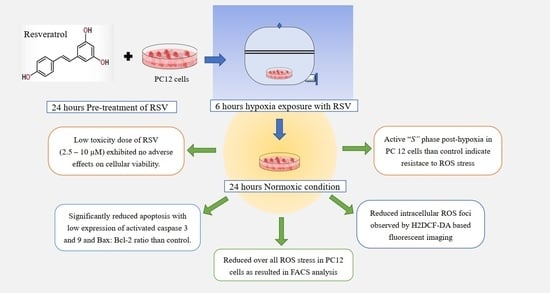
Protective Effect of Resveratrol against Hypoxia-Induced Neural Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture, Resveratrol Treatment, and Hypoxia
2.2. Cytotoxicity Assay
2.3. Annexin V Assay
2.4. Reactive Oxygen Species Detection
2.5. Cell Cycle Analysis
2.6. Western Blotting
2.7. Statistical Analysis
3. Results
3.1. Hypoxia-Induced Cellular Toxicity
3.2. RSV Attenuates Hypoxia-Induced Cell Apoptosis in PC12 Cells
3.3. Antioxidant Effect of RSV on Intracellular ROS
3.4. Measurement of Intracellular ROS Levels
3.5. Cell Cycle Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Domenico, M.; Pinto, F.; Quagliuolo, L.; Contaldo, M.; Settembre, G.; Romano, A.; Coppola, M.; Ferati, K.; Bexheti-Ferati, A.; Sciarra, A.; et al. The Role of Oxidative Stress and Hormones in Controlling Obesity. Front. Endocrinol. 2019, 10, 540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanacore, D.; Messina, G.; Lama, S.; Bitti, G.; Ambrosio, P.; Tenore, G.; Messina, A.; Monda, V.; Zappavigna, S.; Boccellino, M.; et al. Effect of restriction vegan diet’s on muscle mass, oxidative status, and myocytes differentiation: A pilot study. J. Cell. Physiol. 2018, 233, 9345–9353. [Google Scholar] [CrossRef] [PubMed]
- Giudice, A.; Montella, M.; Boccellino, M.; Crispo, A.; D’Arena, G.; Bimonte, S.; Facchini, G.; Ciliberto, G.; Botti, G.; Quagliuolo, L.; et al. Epigenetic Changes Induced by Green Tea Catechins are Associated with Prostate Cancer. Curr. Mol. Med. 2017, 17, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Saberi-Karimian, M.; Katsiki, N.; Caraglia, M.; Boccellino, M.; Majeed, M.; Sahebkar, A. Vascular endothelial growth factor: An important molecular target of curcumin. Crit. Rev. Food Sci. Nutr. 2017, 59, 299–312. [Google Scholar] [CrossRef]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef] [Green Version]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative Stress in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 4094–4125. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Heijnen, C.J.; van der Kooij, M.A.; Groenendaal, F.; van Bel, F. The role and regulation of hypoxia-inducible factor-1α expression in brain development and neonatal hypoxic–ischemic brain injury. Brain Res. Rev. 2009, 62, 99–108. [Google Scholar] [CrossRef]
- Giaccia, A.; Siim, B.G.; Johnson, R.S. HIF-1 as a target for drug development. Nat. Rev. Drug Discov. 2003, 2, 803–811. [Google Scholar] [CrossRef]
- Speer, R.E.; Karuppagounder, S.S.; Basso, M.; Sleiman, S.F.; Kumar, A.; Brand, D.; Smirnova, N.; Gazaryan, I.; Khim, S.J.; Ratan, R.R. Hypoxia-inducible factor prolyl hydroxylases as targets for neuroprotection by “antioxidant” metal chelators: From ferroptosis to stroke. Free Radic. Biol. Med. 2013, 62, 26–36. [Google Scholar] [CrossRef]
- Malthankar-Phatak, G.H.; Patel, A.B.; Xia, Y.; Hong, S.; Chowdhury, G.M.; Behar, K.L.; Orina, I.A.; Lai, J.C. Effects of continuous hypoxia on energy metabolism in cultured cerebro-cortical neurons. Brain Res. 2008, 1229, 147–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurk, D.; Wang, C.; Miwa, S.; Maddick, M.; Korolchuk, V.; Tsolou, A.; Gonos, E.S.; Thrasivoulou, C.; Jill Saffrey, M.; Cameron, K.; et al. Postmitotic neurons develop a p21-dependent senescence-like phenotype driven by a DNA damage response. Aging Cell 2012, 11, 996–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gozal, E.; Metz, C.J.; Dematteis, M.; Sachleben, L.R., Jr.; Schurr, A.; Rane, M.J. PKA activity exacerbates hypoxia-induced ROS formation and hypoxic injury in PC-12 cells. Toxicol. Lett. 2017, 279, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Arrigoni, R.; Ballini, A.; Santacroce, L.; Cantore, S.; Inchingolo, A.; Inchingolo, F.; Di Domenico, M.; Quagliuolo, L.; Boccellino, M. Another look at dietary polyphenols: Challenges in cancer prevention and treatment. Curr. Med. Chem. 2021, 29, 1061–1082. [Google Scholar] [CrossRef]
- Ballini, A.; Scacco, S.; Boccellino, M.; Santacroce, L.; Arrigoni, R. Microbiota and Obesity: Where Are We Now? Biology 2020, 9, 415. [Google Scholar] [CrossRef]
- Di Domenico, M.; Feola, A.; Ambrosio, P.; Pinto, F.; Galasso, G.; Zarrelli, A.; Di Fabio, G.; Porcelli, M.; Scacco, S.; Inchingolo, F.; et al. Antioxidant Effect of Beer Polyphenols and Their Bioavailability in Dental-Derived Stem Cells (D-dSCs) and Human Intestinal Epithelial Lines (Caco-2) Cells. Stem Cells Int. 2020, 2020, 8835813. [Google Scholar] [CrossRef]
- Crocetto, F.; Boccellino, M.; Barone, B.; Di Zazzo, E.; Sciarra, A.; Galasso, G.; Settembre, G.; Quagliuolo, L.; Imbimbo, C.; Boffo, S.; et al. The Crosstalk between Prostate Cancer and Microbiota Inflammation: Nutraceutical Products Are Useful to Balance This Interplay? Nutrients 2020, 12, 2648. [Google Scholar] [CrossRef]
- Ricci, S.; Pinto, F.; Auletta, A.; Giordano, A.; Giovane, A.; Settembre, G.; Boccellino, M.; Boffo, S.; Di Carlo, A.; Di Domenico, M. The enigmatic role of matrix metalloproteinases in epithelial-to-mesenchymal transition of oral squamous cell carcinoma: Implications and nutraceutical aspects. J. Cell. Biochem. 2019, 120, 6813–6819. [Google Scholar] [CrossRef]
- Bandiwadekar, A.; Jose, J.; Khayatkashani, M.; Habtemariam, S.; Khayat Kashani, H.R.; Nabavi, S.M. Emerging Novel Approaches for the Enhanced Delivery of Natural Products for the Management of Neurodegenerative Diseases. J. Mol. Neurosci. 2021, 72, 653–676. [Google Scholar] [CrossRef]
- Franciosoa, A.; Mastromarino, P.; Masci, A.; D’Erme, M.; Mosca, L. Chemistry, Stability and Bioavailability of Resveratrol. Med. Chem. 2014, 10, 237–245. [Google Scholar] [CrossRef]
- Boccellino, M.; D’Angelo, S. Anti-Obesity Effects of Polyphenol Intake: Current Status and Future Possibilities. Int. J. Mol. Sci. 2020, 21, 5642. [Google Scholar] [CrossRef] [PubMed]
- Boccellino, M.; Donniacuo, M.; Bruno, F.; Rinaldi, B.; Quagliuolo, L.; Ambruosi, M.; Pace, S.; De Rosa, M.; Olgaç, A.; Banoglu, E.; et al. Protective effect of piceatannol and bioactive stilbene derivatives against hypoxia-induced toxicity in H9c2 cardiomyocytes and structural elucidation as 5-LOX inhibitors. Eur. J. Med. Chem. 2019, 180, 637–647. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.; La Porta, R.; Napolitano, M.; Galletti, P.; Quagliuolo, L.; Boccellino, M. Effect of Annurca Apple Polyphenols on Human HaCaT Keratinocytes Proliferation. J. Med. Food 2012, 15, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.G.; Mattheos, A.G.K. Bioavailability and Recent Advances in the Bioactivity of Flavonoid and Stilbene Compounds. Curr. Org. Chem. 2010, 14, 1727–1751. [Google Scholar] [CrossRef]
- Cottart, C.-H.; Nivet-Antoine, V.; Beaudeux, J.-L. Review of recent data on the metabolism, biological effects, and toxicity of resveratrol in humans. Mol. Nutr. Food Res. 2013, 58, 7–21. [Google Scholar] [CrossRef]
- Wei, H.; Wang, S.; Zhen, L.; Yang, Q.; Wu, Z.; Lei, X.; Lv, J.; Xiong, L.; Xue, R. Resveratrol Attenuates the Blood-Brain Barrier Dysfunction by Regulation of the MMP-9/TIMP-1 Balance after Cerebral Ischemia Reperfusion in Rats. J. Mol. Neurosci. 2014, 55, 872–879. [Google Scholar] [CrossRef]
- Sun, A.Y.; Wang, Q.; Simonyi, A.; Sun, G.Y. Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol. Neurobiol. 2010, 41, 375–383. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Gerevini, G.T.; Repossi, G.; Dain, A.; Tarres, M.C.; Das, U.N.; Eynard, A.R. Beneficial action of resveratrol: How and why? Nutrition 2016, 32, 174–178. [Google Scholar] [CrossRef]
- Boccellino, M.; Galasso, G.; Ambrosio, P.; Stiuso, P.; Lama, S.; Di Zazzo, E.; Schiavon, S.; Vecchio, D.; D’Ambrosio, L.; Quagliuolo, L.; et al. H9c2 Cardiomyocytes under Hypoxic Stress: Biological Effects Mediated by Sentinel Downstream Targets. Oxidative Med. Cell. Longev. 2021, 2021, 6874146. [Google Scholar] [CrossRef]
- Boccellino, M.; Di Domenico, M.; Donniacuo, M.; Bitti, G.; Gritti, G.; Ambrosio, P.; Quagliuolo, L.; Rinaldi, B. AT1-receptor blockade: Protective effects of irbesartan in cardiomyocytes under hypoxic stress. PLoS ONE 2018, 13, e0202297. [Google Scholar] [CrossRef] [Green Version]
- Spugnini, E.P.; Melillo, A.; Quagliuolo, L.; Boccellino, M.; Vincenzi, B.; Pasquali, P.; Baldi, A. Definition of novel electrochemotherapy parameters and validation of their in vitro and in vivo effectiveness. J. Cell. Physiol. 2014, 229, 1177–1181. [Google Scholar] [CrossRef] [PubMed]
- Boccellino, M.; La Porta, R.; Coppola, M.; Petronella, P.; Freda, F.; Calderaro, V.; Quagliuolo, L. Peritoneal dialysis fluid activates calcium signaling and apoptosis in mesothelial cells. Apoptosis 2013, 18, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Aprile, D.; Alessio, N.; Demirsoy, I.H.; Squillaro, T.; Peluso, G.; Di Bernardo, G.; Galderisi, U. MUSE Stem Cells Can Be Isolated from Stromal Compartment of Mouse Bone Marrow, Adipose Tissue, and Ear Connective Tissue: A Comparative Study of Their In Vitro Properties. Cells 2021, 10, 761. [Google Scholar] [CrossRef] [PubMed]
- Alessio, N.; Capasso, S.; Di Bernardo, G.; Cappabianca, S.; Casale, F.; Calarco, A.; Cipollaro, M.; Peluso, G.; Galderisi, U. Mesenchymal stromal cells having inactivated RB1 survive following low irradiation and accumulate damaged DNA: Hints for side effects following radiotherapy. Cell Cycle 2017, 16, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Boccellino, M.; Camussi, G.; Giovane, A.; Ferro, L.; Calderaro, V.; Balestrieri, C.; Quagliuolo, L. Platelet-activating factor regulates cadherin-catenin adhesion system expression and beta-catenin phosphorylation during Kaposi’s sarcoma cell motility. Am. J. Pathol. 2005, 166, 1515–1522. [Google Scholar] [CrossRef]
- Buommino, E.; Boccellino, M.; De Filippis, A.; Petrazzuolo, M.; Cozza, V.; Nicoletti, R.; Ciavatta, M.L.; Quagliuolo, L.; Tufano, M.A. 3-O-methylfunicone produced by penicillium pinophilum affects cell motility of breast cancer cells, downregulating alphavbeta5 integrin and inhibiting metalloproteinase-9 secretion. Mol. Carcinog. 2007, 46, 930–940. [Google Scholar] [CrossRef]
- Boccellino, M.; Cuccovillo, F.; Napolitano, M.; Sannolo, N.; Balestrieri, C.; Acampora, A.; Giovane, A.; Quagliuolo, L. Styrene-7,8-oxide activates a complex apoptotic response in neuronal PC12 cell line. Carcinogenesis 2003, 24, 535–540. [Google Scholar] [CrossRef] [Green Version]
- Robb, E.L.; Page, M.M.; Wiens, B.E.; Stuart, J.A. Molecular mechanisms of oxidative stress resistance induced by resveratrol: Specific and progressive induction of MnSOD. Biochem. Biophys. Res. Commun. 2008, 367, 406–412. [Google Scholar] [CrossRef]
- Quoc Trung, L.; Espinoza, J.L.; Takami, A.; Nakao, S. Resveratrol induces cell cycle arrest and apoptosis in malignant NK cells via JAK2/STAT3 pathway inhibition. PLoS ONE 2013, 8, e55183. [Google Scholar] [CrossRef] [Green Version]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef] [Green Version]
- Müller, G.J.; Stadelmann, C.; Bastholm, L.; Elling, F.; Lassmann, H.; Johansen, F.F. Ischemia leads to apoptosis-and necrosis-like neuron death in the ischemic rat hippocampus. Brain Pathol. 2004, 14, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Zivin, J.A. Factors determining the therapeutic window for stroke. Neurology 1998, 50, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Jaffer, H.; Morris, V.B.; Stewart, D.; Labhasetwar, V. Advances in stroke therapy. Drug Deliv. Transl. Res. 2011, 1, 409–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Botchway, B.O.; Tan, X.; Zhang, Y.; Fang, M. Resveratrol treatment of spinal cord injury in rat model. Microsc. Res. Tech. 2019, 82, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chen, S.; Gao, K.; Zhou, Z.; Wang, C.; Shen, Z.; Guo, Y.; Li, Z.; Wan, Z.; Liu, C.; et al. Resveratrol protects against spinal cord injury by activating autophagy and inhibiting apoptosis mediated by the SIRT1/AMPK signaling pathway. Neuroscience 2017, 348, 241–251. [Google Scholar] [CrossRef]
- Wang, D.; Li, S.P.; Fu, J.S.; Zhang, S.; Bai, L.; Guo, L. Resveratrol defends blood-brain barrier integrity in experimental autoimmune encephalomyelitis mice. J. Neurophysiol. 2016, 116, 2173–2179. [Google Scholar] [CrossRef] [Green Version]
- Mukandala, G.; Tynan, R.; Lanigan, S.; O’Connor, J.J. The Effects of Hypoxia and Inflammation on Synaptic Signaling in the CNS. Brain Sci. 2016, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Huang, J.; Shen, C.; Cheng, W.; Yu, P.; Wang, L.; Tang, F.; Guo, S.; Yang, Q.; Zhang, J. Resveratrol Treatment in Different Time-Attenuated Neuronal Apoptosis After Oxygen and Glucose Deprivation/Reoxygenation via Enhancing the Activation of Nrf-2 Signaling Pathway In Vitro. Cell Transplant. 2018, 27, 1789–1797. [Google Scholar] [CrossRef]
- Hamad, I.; Arda, N.; Pekmez, M.; Karaer, S.; Temizkan, G. Intracellular scavenging activity of Trolox (6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid) in the fission yeast, Schizosaccharomyces pombe. J. Nat. Sci. Biol. Med. 2010, 1, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Wu, M.H.; Dong, J.; Liu, N.; Li, J.M.; Zhang, J.X.; Li, S.Y.; Wang, R.M.; Chen, G.L. Neuronal differentiation of adipose tissue-derived stromal cells. Chin. J. Tissue Eng. Res. 2010, 14, 15–18. [Google Scholar]
- Zhang, Q. Summarize of cell apoptosis mechanism. Environ. Occup. Med. 2007, 24, 102–107. [Google Scholar]
- Zhang, L.; Yuan, X.; Wang, S.; Ou, Y.; Zheng, X.; Wang, Q. The relationship between mitochondrial fusion/fission and apoptosis in the process of adipose-derived stromal cells differentiation into astrocytes. Neurosci. Lett. 2014, 575, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Akyuva, Y.; Nazıroğlu, M. Resveratrol attenuates hypoxia-induced neuronal cell death, inflammation and mitochondrial oxidative stress by modulation of TRPM2 channel. Sci. Rep. 2020, 10, 6449. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auti, A.; Alessio, N.; Ballini, A.; Dioguardi, M.; Cantore, S.; Scacco, S.; Vitiello, A.; Quagliuolo, L.; Rinaldi, B.; Santacroce, L.; et al. Protective Effect of Resveratrol against Hypoxia-Induced Neural Oxidative Stress. J. Pers. Med. 2022, 12, 1202. https://doi.org/10.3390/jpm12081202
Auti A, Alessio N, Ballini A, Dioguardi M, Cantore S, Scacco S, Vitiello A, Quagliuolo L, Rinaldi B, Santacroce L, et al. Protective Effect of Resveratrol against Hypoxia-Induced Neural Oxidative Stress. Journal of Personalized Medicine. 2022; 12(8):1202. https://doi.org/10.3390/jpm12081202
Chicago/Turabian StyleAuti, Amogh, Nicola Alessio, Andrea Ballini, Mario Dioguardi, Stefania Cantore, Salvatore Scacco, Antonio Vitiello, Lucio Quagliuolo, Barbara Rinaldi, Luigi Santacroce, and et al. 2022. "Protective Effect of Resveratrol against Hypoxia-Induced Neural Oxidative Stress" Journal of Personalized Medicine 12, no. 8: 1202. https://doi.org/10.3390/jpm12081202
APA StyleAuti, A., Alessio, N., Ballini, A., Dioguardi, M., Cantore, S., Scacco, S., Vitiello, A., Quagliuolo, L., Rinaldi, B., Santacroce, L., Di Domenico, M., & Boccellino, M. (2022). Protective Effect of Resveratrol against Hypoxia-Induced Neural Oxidative Stress. Journal of Personalized Medicine, 12(8), 1202. https://doi.org/10.3390/jpm12081202