Molecular Bases of Serotonin Reuptake Inhibitor Antidepressant-Attributed Effects in COVID-19: A New Insight on the Role of Bradykinins
Abstract
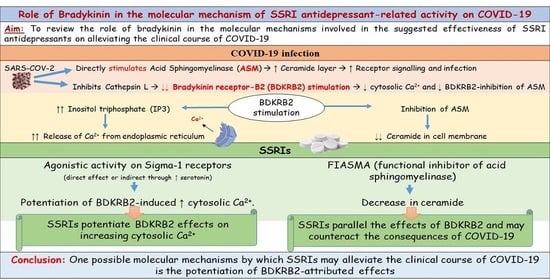
:1. Introduction
2. SSRI Antidepressant-Mediated Effects on the Sigma-1 Receptor in COVID-19
2.1. The Non-Opioid Sigma-1 Receptor
2.2. Implication in SARS-CoV-2 Infection
2.3. Interaction with the Kinin-Kallikreine System
3. SSRI Antidepressant-Mediated Effects on the ASM/Ceramide System in COVID-19
3.1. The ASM/Ceramide System
3.2. Implication in SARS-CoV-2 Infection
3.3. Interaction with the Kinin-Kallikreine System
4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 16 August 2022).
- Alam, S.; Kamal, T.B.; Sarker, M.M.R.; Zhou, J.R.; Rahman, S.M.A.; Mohamed, I.N. Therapeutic Effectiveness and Safety of Repurposing Drugs for the Treatment of COVID-19: Position Standing in 2021. Front. Pharmacol. 2021, 12, 659577. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.T.; Malani, P.N.; Del Rio, C. COVID-19 Therapeutics for Nonhospitalized Patients. JAMA 2022, 327, 617–618. [Google Scholar] [CrossRef] [PubMed]
- Barcella, C.A.; Polcwiartek, C.; Mohr, G.H.; Hodges, G.; Søndergaard, K.; Niels Bang, C.; Andersen, M.P.; Fosbøl, E.; Køber, L.; Schou, M.; et al. Severe mental illness is associated with increased mortality and severe course of COVID-19. Acta Psychiatr. Scand. 2021, 144, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Diez-Quevedo, C.; Iglesias-González, M.; Giralt-López, M.; Rangil, T.; Sanagustin, D.; Moreira, M.; López-Ramentol, M.; Ibáñez-Caparrós, A.; Lorán, M.E.; Bustos-Cardona, T.; et al. Mental disorders, psychopharmacological treatments, and mortality in 2150 COVID-19 Spanish inpatients. Acta Psychiatr. Scand. 2021, 143, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Hoertel, N.; Sánchez-Rico, M.; Vernet, R.; Beeker, N.; Jannot, A.S.; Neuraz, A.; Salamanca, E.; Paris, N.; Daniel, C.; Gramfort, A.; et al. Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID-19: Results from an observational study. Mol. Psychiatry 2021, 26, 5199–5212. [Google Scholar] [CrossRef] [PubMed]
- Hoertel, N.; Sánchez-Rico, M.; Gulbins, E.; Kornhuber, J.; Carpinteiro, A.; Lenze, E.J.; Reiersen, A.M.; Abellán, M.; de la Muela, P.; Vernet, R.; et al. Association Between FIASMAs and Reduced Risk of Intubation or Death in Individuals Hospitalized for Severe COVID-19: An Observational Multicenter Study. Clin. Pharmacol. Therap. 2021, 110, 1498–1511. [Google Scholar] [CrossRef]
- Oskotsky, T.; Maric, I.; Tang, A.; Oskotsky, B.; Wong, R.J.; Aghaeepour, N.; Sirota, M.; Stevenson, D.K. Mortality Risk Among Patients With COVID-19 Prescribed Selective Serotonin Reuptake Inhibitor Antidepressants. JAMA Netw. Open 2021, 4, e2133090. [Google Scholar] [CrossRef]
- Fei, L.; Santarelli, G.; D’Anna, G.; Moretti, S.; Mirossi, G.; Patti, A.; Sanfilippo, G.; Almerigogna, F.; Berni, A.; Caldini, E.; et al. Can SSRI/SNRI antidepressants decrease the ‘cytokine storm’ in the course of COVID-19 pneumonia? Panminerva Med. 2021. [Google Scholar] [CrossRef]
- Németh, Z.K.; Szûcs, A.; Vitrai, J.; Juhász, D.; Németh, J.P.; Holló, A. Fluoxetine use is associated with improved survival of patients with COVID-19 pneumonia: A retrospective case-control study. Ideggyogy Sz. 2021, 74, 389–396. [Google Scholar] [CrossRef]
- Bonnet, U.; Claus, B.; Schaefer, M.; Kuhn, J.; Nyhuis, P.; Scherbaum, N.; Brüne, M.; Wakili, V.; Juckel, G. Impact of Psychiatric and Related Somatic Medications on the Duration and Severity of COVID-19: A Retrospective Explorative Multi-center Study from the German Metropolitan Ruhr-area. Pharmacopsychiatry 2022, 55, 30–39. [Google Scholar] [CrossRef]
- Clelland, C.L.; Ramiah, K.; Steinberg, L.; Clelland, J.D. Analysis of the impact of antidepressants and other medications on COVID-19 infection risk in a chronic psychiatric in-patient cohort. BJPsych. Open 2021, 8, e6. [Google Scholar] [CrossRef] [PubMed]
- Seftel, D.; Boulware, D.R. Prospective Cohort of Fluvoxamine for Early Treatment of Coronavirus Disease 19. Open Forum Infect. Dis. 2021, 8, ofab050. [Google Scholar] [CrossRef] [PubMed]
- Calusic, M.; Marcec, R.; Luksa, L.; Jurkovic, I.; Kovac, N.; Mihaljevic, S.; Likic, R. Safety and efficacy of fluvoxamine in COVID-19 ICU patients: An open label, prospective cohort trial with matched controls. Br. J. Clin. Pharmacol. 2022, 88, 2065–2073. [Google Scholar] [CrossRef] [PubMed]
- Lenze, E.J.; Mattar, C.; Zorumski, C.F.; Stevens, A.; Schweiger, J.; Nicol, G.E.; Miller, J.P.; Yang, L.; Yingling, M.; Avidan, M.S.; et al. Fluvoxamine vs Placebo and Clinical Deterioration in Outpatients with Symptomatic COVID-19. JAMA 2020, 324, 2292. [Google Scholar] [CrossRef] [PubMed]
- Reis, G.; Dos Santos Moreira-Silva, E.A.; Silva, D.C.M.; Thabane, L.; Milagres, A.C.; Ferreira, T.S.; Dos Santos, C.V.Q.; de Souza Campos, V.H.; Nogueira, A.M.R.; de Almeida, A.P.F.G.; et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: The TOGETHER randomised, platform clinical trial. Lancet Glob. Health 2022, 10, e42–e51. [Google Scholar] [CrossRef]
- Lenze, E. Fluvoxamine for Early Treatment of COVID-19: A Fully-Remote, Randomized Placebo Controlled Trial. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04668950 (accessed on 5 August 2022).
- Bhuta, S.; Khokher, W.; Kesireddy, N.; Iftikhar, S.; Beran, A.; Mhanna, M.; Patel, N.J.; Patel, M.; Burmeister, C.; Assaly, R. Fluvoxamine in Nonhospitalized Patients with Acute COVID-19 Infection and the Lack of Efficacy in Reducing Rates of Hospitalization, Mechanical Ventilation, and Mortality in Placebo-Controlled Trials: A Systematic Review and Meta-Analysis. Am. J. Ther. 2022, 29, e298–e304. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.C.; Vigod, S.; Bortolussi-Courval, É.; Hanula, R.; Boulware, D.R.; Lenze, E.J.; Reiersen, A.M.; McDonald, E.G. Fluvoxamine for Outpatient Management of COVID-19 to Prevent Hospitalization: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e226269. [Google Scholar] [CrossRef]
- Guo, C.M.; Harari, O.; Chernecki, C.; Thorlund, K.; Forrest, J.I. Fluvoxamine for the Early Treatment of COVID-19: A Meta-analysis of Randomized Clinical Trials. Am. J. Trop. Med. Hyg. 2022, 106, 1315–1320. [Google Scholar] [CrossRef]
- Trkulja, V. Fluvoxamine for COVID-19 outpatients: For the time being, we might prefer to curb our optimism. Br. J. Clin. Pharmacol. 2022, 88, 4654–4656. [Google Scholar] [CrossRef]
- Trkulja, V. Fluvoxamine for COVID-19 ICU patients? Br. J. Clin. Pharmacol. 2022, 88, 2454–2455. [Google Scholar] [CrossRef]
- Li, J.R.; Xu, H.Z.; Nie, S.; Peng, Y.C.; Fan, L.F.; Wang, Z.J.; Wu, C.; Yan, F.; Chen, J.Y.; Gu, C.; et al. Fluoxetine-enhanced autophagy ameliorates early brain injury via inhibition of NLRP3 inflammasome activation following subrachnoid hemorrhage in rats. J. Neuroinflam. 2017, 14, 186. [Google Scholar] [CrossRef] [PubMed]
- Gouda, A.S.; Mégarbane, B. Snake venom-derived bradykinin-potentiating peptides: A promising therapy for COVID-19? Drug Dev. Res. 2021, 82, 38–48. [Google Scholar] [CrossRef] [PubMed]
- van de Veerdonk, F.L.; Netea, M.G.; van Deuren, M.; van der Meer, J.W.; de Mast, Q.; Brüggemann, R.J.; van der Hoeven, H. Kallikrein-kinin blockade in patients with COVID-19 to prevent acute respiratory distress syndrome. Elife 2020, 9, e57555. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Su, T.P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca2+ signaling and cell survival. Cell 2007, 131, 596–610. [Google Scholar] [CrossRef]
- Hayashi, T.; Su, T.P. Regulating ankyrin dynamics: Roles of sigma-1 receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 491–496. [Google Scholar] [CrossRef]
- Hayashi, T. The Sigma-1 Receptor in Cellular Stress Signaling. Front. Neurosci. 2019, 13, 733. [Google Scholar] [CrossRef]
- Wu, Z.; Bowen, W.D. Role of Sigma-1 Receptor C-terminal Segment in Inositol 1,4,5-Trisphosphate Receptor Activation. J. Biol. Chem. 2008, 283, 28198–28215. [Google Scholar] [CrossRef]
- Brimson, J.M.; Prasanth, M.I.; Malar, D.S.; Brimson, S.; Thitilertdecha, P.; Tencomnao, T. Drugs that offer the potential to reduce hospitalization and mortality from SARS-CoV-2 infection: The possible role of the sigma-1 receptor and autophagy. Expert Opin. Ther. Targets 2021, 25, 435–449. [Google Scholar] [CrossRef]
- Vela, J.M. Repurposing Sigma-1 Receptor Ligands for COVID-19 Therapy? Front. Pharmacol. 2020, 11, 582310. [Google Scholar] [CrossRef]
- Tsai, S.Y.; Pokrass, M.J.; Klauer, N.R.; De Credico, N.E.; Su, T.P. Sigma-1 receptor chaperones in neurodegenerative and psychiatric disorders. Expert Opin. Ther. Targets 2014, 18, 1461–1476. [Google Scholar] [CrossRef] [Green Version]
- Gordon, D.E.; Hiatt, J.; Bouhaddou, M.; Rezelj, V.V.; Ulferts, S.; Braberg, H.; Jureka, A.S.; Obernier, K.; Guo, J.Z.; Batra, J.; et al. Comparative host-coronavirus protein interaction networks reveal pan-viral disease mechanisms. Science 2020, 370, eabe9403. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, S.; Rheinstein, P.H. Homozygosity for rs17775810 Minor Allele Associated with Reduced Mortality of COVID-19 in the UK Biobank Cohort. In Vivo 2021, 35, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Shen, H.; Li, J.; Guo, L.W. SIGMAR1/Sigma-1 receptor ablation impairs autophagosome clearance. Autophagy 2019, 15, 1539–1557. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Sun, Y.; Sun, X.; Zhou, Y.; Bian, Y.; Shu, Z.; Ding, J.; Lu, M.; Hu, G. The effect of fluoxetine on astrocyte autophagy flux and injured mitochondria clearance in a mouse model of depression. Cell Death Dis. 2019, 10, 577. [Google Scholar] [CrossRef]
- Marsh, K.A.; Hill, S.J. Characteristics of the bradykinin-induced changes in intracellular calcium ion concentration of single bovine tracheal smooth muscle cells. Br. J. Pharmacol. 1993, 110, 29–35. [Google Scholar] [CrossRef]
- Ricciardolo, F.L.; Lovett, M.; Halliday, D.A.; Nadel, J.A.; Kaneko, T.; Bunnett, N.W.; Geppetti, P. Bradykinin increases intracellular calcium levels in a human bronchial epithelial cell line via the B 2 receptor subtype. Inflam. Res. 1998, 47, 231–235. [Google Scholar] [CrossRef]
- Dickenson, J.M.; Bulmer, S.; Whittaker, A.; Salwey, M.; Hawley, J.; Hill, S.J. Bradykinin B2- and 5-hydroxytryptamine (5-HT2)-receptor stimulated increases in intracellular calcium in cultured guinea-pig aortic smooth muscle cells. Biochem. Pharmacol. 1994, 47, 947–952. [Google Scholar] [CrossRef]
- Sangkuhl, K.; Klein, T.E.; Altman, R.B. Selective serotonin reuptake inhibitors pathway. Pharmacogenet. Genom. 2009, 19, 907–909. [Google Scholar] [CrossRef]
- McCloskey, D.J.; Postolache, T.T.; Vittone, B.J.; Nghiem, K.L.; Monsale, J.L.; Wesley, R.A.; Rick, M.E. Selective serotonin reuptake inhibitors: Measurement of effect on platelet function. Transl. Res. 2008, 151, 168–172. [Google Scholar] [CrossRef]
- Gurusamy, M.; Nasseri, S.; Lee, H.; Jung, B.; Lee, D.; Khang, G.; Abraham, W.M.; Doods, H.; Wu, D. Kinin B1 receptor antagonist BI113823 reduces allergen-induced airway inflammation and mucus secretion in mice. Pharmacol. Res. 2016, 104, 132–139. [Google Scholar] [CrossRef]
- Soria-Castro, R.; Meneses-Preza, Y.G.; Rodríguez-López, G.M.; Romero-Ramírez, S.; Sosa-Hernández, V.A.; Cervantes-Díaz, R.; Pérez-Fragoso, A.; Torres-Ruíz, J.J.; Gómez-Martín, D.; Campillo-Navarro, M.; et al. Severe COVID-19 is marked by dysregulated serum levels of carboxypeptidase A3 and serotonin. J. Leukoc. Biol. 2021, 110, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Carpinteiro, A.; Edwards, M.J.; Hoffmann, M.; Kochs, G.; Gripp, B.; Weigang, S.; Adams, C.; Carpinteiro, E.; Gulbins, A.; Keitsch, S.; et al. Pharmacological Inhibition of Acid Sphingomyelinase Prevents Uptake of SARS-CoV-2 by Epithelial Cells. Cell Rep. Med. 2020, 1, 100142. [Google Scholar] [CrossRef] [PubMed]
- Siskind, L.J.; Mullen, T.D.; Obeid, L.M. The Role of Ceramide in Cell Regulation. In Handbook of Cell Signalling, 2nd ed.; Bradshaw, R., Dennis, E., Eds.; Elsevier: Amsterdam, The Netherlands; Academic Press: California, CA, USA, 2009; pp. 1201–1211. [Google Scholar]
- Abusukhun, M.; Winkler, M.S.; Pöhlmann, S.; Moerer, O.; Meissner, K.; Tampe, B.; Hofmann-Winkler, H.; Bauer, M.; Gräler, M.H.; Claus, R.A. Activation of Sphingomyelinase-Ceramide-Pathway in COVID-19 Purposes Its Inhibition for Therapeutic Strategies. Front. Immunol. 2021, 12, 784989. [Google Scholar] [CrossRef] [PubMed]
- Carpinteiro, A.; Gripp, B.; Hoffmann, M.; Pöhlmann, S.; Hoertel, N.; Edwards, M.J.; Kamler, M.; Kornhuber, J.; Becker, K.A.; Gulbins, E. Inhibition of acid sphingomyelinase by ambroxol prevents SARS-CoV-2 entry into epithelial cells. J. Biol. Chem. 2021, 296, 100701. [Google Scholar] [CrossRef]
- Liu, G.; Kleine, L.; Nasrallah, R.; Hébert, R.L. Bradykinin inhibits ceramide production and activates phospholipase D in rabbit cortical collecting duct cells. Am. J. Physiol. 1999, 276, F589–F598. [Google Scholar] [CrossRef]
- Kleine, L.; Liu, G.; Leblanc, N.; Hébert, R.L. Bradykinin stimulates ceramide production by activating specific BK-B(1) receptor in rat small artery. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H175–H183. [Google Scholar] [CrossRef] [Green Version]

| No. | Study Design | Tested Molecules | Dose Regimen | Subjects | Findings | Flaws and Limitatios |
|---|---|---|---|---|---|---|
| [4] | Cross-sectional nationwide registry study | All psychotropic medications | Not defined | N = 144,321 patients | No significant association between the number of psychotropic drugs and the higher risk of severe COVID or death. | No differentiation in psychotropic drugs; heterogeneous data; no control group. Not contributive. |
| [5] | Retrospective observational single-center cohort study | All psychotropic medications | Not defined | N = 2150 patients | Independent association of previous year’s treatments with anxiolytics/hypnotics and antidepressants with lower mortality risk (HR, 0.47 and 0.43, respectively). | No differentiation in psychotropic drugs; heterogeneous data; no control group. Not contributive. |
| [6] | Retrospective observational multicenter cohort study | All antidepressants | Not defined | N = 345 treated patients among 7230 patients | Significant association between antidepressant use and reduced risk of intubation or death (HR, 0.56; CI, 0.43–0.73; p < 0.001). Significant association for SSRI and non-SSRI antidepressants, and for fluoxetine, paroxetine, escitalopram, venlafaxine, and mirtazapine (all p < 0.05). | No differentiation in antidepressants; heterogeneous data (indications, dosage, compliance, duration); no control group. Little contributive. |
| [7] | Retrospective observational multicenter cohort study | FIASMA medications | Not defined | N = 277 treated patients among 2846 patients | Significant association of FIASMA medication use with reduced likelihood of intubation or death in both crude (HR, 0.71; CI, 0.58–0.87; p < 0.001) and primary inverse probability weighting (HR, 0.58; CI, 0.46–0.72; p < 0.001) analyses. Significant association in multiple sensitivity analyses, not specific to one particular FIASMA class or medication. | No differentiation in FIASMA medications; heterogeneous data (indications, dosage, compliance, duration); no control group. Little contributive. |
| [8] | Multicenter retrospective cohort study with propensity score matching | Fluoxetine, fluvoxamine, or other SSRI | Not defined | N = 3401 patients | When compared with matched untreated control patients, reduction of mortality among patients prescribed any SSRI (RR, 0.92; CI, 0.85–0.99; p = 0.03); fluoxetine (RR, 0.72; CI, 0.54–0.97; p = 0.03); and fluoxetine or fluvoxamine (RR, 0.74; CI, 0.55–0.99; p = 0.04). No significant association between receiving any SSRI that is not fluoxetine or fluvoxamine and risk of death (RR, 0.92; CI, 0.84–1.00; p = 0.06). | Heterogeneous data (indications, dosage, compliance, duration); no control group. Little contributive. |
| [9] | Single-center retrospective case-control study | All antidepressants | Not defined | N = 34 treated vs. 368 non-treated patients | Reduction in ARDS development (p < 0.02) and tracheal intubation (p = 0.04). No significant reduction in mortality rate. | Small sample size; heterogeneous data (indications, dosage, compliance, duration); differences between the 2 groups (comorbidities and antiviral therapies) no control group. Not contributive. |
| [10] | Single-center retrospective case-control study | Fluvoxetine vs. observation alone | Fluoxetine 20 mg once daily | N = 269 patients (110 treated vs. 159 not treated) | Significant decrease in mortality (OR = 0.33; CI, 0.16–0.68; p = 0.002). Three cases with adverse effects requiring cessation of fluoxetine. | Small sample size; differences between the 2 groups (antiviral therapies). Little contributive. |
| [11] | Retrospective longitudinal, multicenter inpatient study | All psychotropic medications | Not defined | N = 96 patients | No tested medication was significantly associated with COVID-19 duration and severity up to the end of post-diagnosing week 3. | Limited sample size; heterogeneous data; loss of follow-up (11%); no control group. Little contributive. |
| [12] | Single-center retrospective case-control study | All antidepressants | Not defined | N = 165 patients | Protective association between antidepressant use and COVID-19 infection (OR = 0.33; CI, 0.15–0.70; p < 0.05). Association between lower risk of infection and fluoxetine (p = 0.023) and trazodone use (p = 0.001). | Limited sample size; no control group; no report of COVID-19 severity and outcome. Not contributive. |
| [13] | Prospective non-randomized comparative study | Fluvoxamine vs. observation alone | Fluvoxamine 50 mg twice daily | N = 98 patients. No statistical analysis | Reduction in incidence of hospitalization (0% vs. 12.5%) and persistence of residual symptoms (0% vs. 60%). | Limited sample size; possible confounders (comorbidities, comedications). Some insufficiencies. |
| [14] | Open-label, prospective cohort trial with matched controls | Fluvoxamine vs. observation alone | Fluvoxamine 100 mg three times daily for 15 days | N = 102 intensive care unit patients | No significant difference regarding the number of days on ventilator support, duration of intensive care unit, or total hospital stay. Reduction in overall mortality (58.8% vs. 76.5%, HR 0.58; CI, 0.36–0.94; p = 0.027). | Limited sample size; unreported matching method and adequacy; baseline between-group imbalance; analysis biases. Important insufficiencies. |
| [15] | Double-blind, randomized, fully remote (contactless) clinical trial | Fluvoxamine vs. placebo | Fluvoxamine 100 mg thrice daily for 15 days | N = 152 patients | Reduction in clinical deterioration (absolute difference, 8.7%; CI, 1.8%–16.4%, log-rank p = 0.009). However, study limitation due to its small sample size and short follow-up duration. | Small sample size; short follow-up; weak outcome criteria; attrition bias (20%). Interesting preliminary investigation. |
| [16] | Placebo-controlled, randomized, adaptive platform trial | Fluvoxamine vs. placebo | Fluvoxamine 100 mg twice daily for 10 days | N = 1497 patients | Reduction in the proportion of patients observed in a COVID-19 emergency setting for >6 h or transferred to a tertiary hospital due to COVID-19 (11% vs. 16%; RR, 0.68; BCI, 0.52–0.88), with a probability of superiority of 99.8% surpassing the pre-specified superiority threshold of 97.6% (risk difference, 5%). Similar findings for the modified intention-to-treat analysis (RR, 0.69; BCI, 0.53–0.90), but larger in the per-protocol analysis (RR, 0.34; BCI, 0.21–0.54). Reduction in mortality in the primary intention-to-treat analysis (OR, 0.68; CI, 0.36–1.27). Reduction in the observed mortality (OR, 0.09; CI, 0.01–0.47). | Possible confounders (comedications). Interesting investigation. |
| SSRI Administration | Bradykinins | COVID-19 Patients | |
|---|---|---|---|
| Blood serotonin level | Increased | ND | Decreased |
| Platelet aggregation | Decreased | BDKRB1: increase BDKRB2: decrease | Increased |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gouda, A.S.; Mégarbane, B. Molecular Bases of Serotonin Reuptake Inhibitor Antidepressant-Attributed Effects in COVID-19: A New Insight on the Role of Bradykinins. J. Pers. Med. 2022, 12, 1487. https://doi.org/10.3390/jpm12091487
Gouda AS, Mégarbane B. Molecular Bases of Serotonin Reuptake Inhibitor Antidepressant-Attributed Effects in COVID-19: A New Insight on the Role of Bradykinins. Journal of Personalized Medicine. 2022; 12(9):1487. https://doi.org/10.3390/jpm12091487
Chicago/Turabian StyleGouda, Ahmed S., and Bruno Mégarbane. 2022. "Molecular Bases of Serotonin Reuptake Inhibitor Antidepressant-Attributed Effects in COVID-19: A New Insight on the Role of Bradykinins" Journal of Personalized Medicine 12, no. 9: 1487. https://doi.org/10.3390/jpm12091487
APA StyleGouda, A. S., & Mégarbane, B. (2022). Molecular Bases of Serotonin Reuptake Inhibitor Antidepressant-Attributed Effects in COVID-19: A New Insight on the Role of Bradykinins. Journal of Personalized Medicine, 12(9), 1487. https://doi.org/10.3390/jpm12091487