Diagnostic Value of the Combined Measurement of Serum HCY and NRG4 in Type 2 Diabetes Mellitus with Early Complicating Diabetic Nephropathy
Abstract
:1. Introduction
2. Materials and Methods
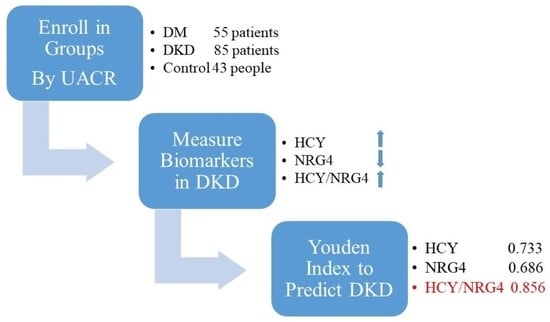
2.1. Selection of Patients and the Research Design
2.2. Data Collection and Definition
2.3. Serum HCY and Neuregulin-4 Measurement
2.4. Statistical Analysis
3. Results
3.1. Clinical Data Characteristics of Patients
3.2. The Predictive Efficacy of HCY, NRG4 and HCY/NRG4 in Predicting DKD
3.3. Analysis of Multiple Factors Affecting DKD Occurrence
3.4. Construction and Clinical Value of Predictive Nomogram
3.5. Pearson Correlation Analysis between Serum HCY/NRG4 Level and Other Indicators
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Diabetes. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 25 May 2022).
- Dekamin, A.; Wahab, M.I.M.; Keshavjee, K.; Guergachi, A. High cardiovascular disease risk-associated with the incidence of Type 2 diabetes among prediabetics. Eur. J. Intern. Med. 2022, 106, 56–62. [Google Scholar] [CrossRef]
- Faselis, C.; Katsimardou, A.; Imprialos, K.; Deligkaris, P.; Kallistratos, M.; Dimitriadis, K. Microvascular Complications of Type 2 Diabetes Mellitus. Curr. Vasc. Pharmacol. 2020, 18, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Viigimaa, M.; Sachinidis, A.; Toumpourleka, M.; Koutsampasopoulos, K.; Alliksoo, S.; Titma, T. Macrovascular Complications of Type 2 Diabetes Mellitus. Curr. Vasc. Pharmacol. 2020, 18, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.; Song, A.; Zhang, C. Modes of podocyte death in diabetic kidney disease: An update. J. Nephrol. 2022, 35, 1571–1584. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Chen, P.P.; Zhang, J.X.; Li, X.Q.; Wang, G.H.; Yuan, B.Y.; Huang, S.J.; Liu, X.Q.; Jiang, T.T.; Wang, M.Y.; et al. GPR43 deficiency protects against podocyte insulin resistance in diabetic nephropathy through the restoration of AMPKα activity. Theranostics 2021, 11, 4728–4742. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.E. Pathogenesis, prevention, and treatment of diabetic nephropathy. Lancet 1998, 352, 213–219. [Google Scholar] [CrossRef]
- Zhang, X.X.; Kong, J.; Yun, K. Prevalence of Diabetic Nephropathy among Patients with Type 2 Diabetes Mellitus in China: A Meta-Analysis of Observational Studies. J. Diabetes Res. 2020, 2020, 2315607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tziomalos, K.; Athyros, V.G. Diabetic Nephropathy: New Risk Factors and Improvements in Diagnosis. Rev. Diabet Stud. 2015, 12, 110–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazzerini, P.E.; Capecchi, P.L.; Selvi, E.; Lorenzini, S.; Bisogno, S.; Galeazzi, M.; Pasini, F.L. Hyperhomocysteinemia: A cardiovascular risk factor in autoimmune diseases? Lupus 2007, 16, 852–862. [Google Scholar] [CrossRef]
- Karabag, T.; Kaya, A.; Temizhan, A.; Koç, F.; Yavuz, S.; Cam, S. The influence of homocysteine levels on endothelial function and their relation with microvascular complications in T2DM patients without macrovascular disease. Acta Diabetol. 2007, 44, 69–75. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Q.; Wang, Z.; Xiao, Z.; Liu, L. Diagnostic value of the combined measurement of serum hcy, serum cys C, and urinary microalbumin in type 2 diabetes mellitus with early complicating diabetic nephropathy. ISRN Endocrinol. 2013, 2013, 407452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, B.; Zhu, X.; Zeng, Z.; Ji, X.; Ji, M. Clinical significance of serum homocysteine as a biomarker for early diagnosis of diabetic nephropathy in type 2 diabetes mellitus patients. Pteridines 2021, 32, 11–16. [Google Scholar] [CrossRef]
- Zhou, X.; Shi, A.; Zhou, X. A meta-analysis of serum Hcy in diagnosis of diabetic nephropathy. Pteridines 2020, 31, 1–8. [Google Scholar] [CrossRef]
- Pfeifer, A. NRG4: An endocrine link between brown adipose tissue and liver. Cell Metab. 2015, 21, 13–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.X.; Zhao, X.Y.; Lin, J.D. The brown fat secretome: Metabolic functions beyond thermogenesis. Trends Endocrinol. Metab. 2015, 26, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Yan, P.; Xu, Y.; Wan, Q.; Feng, J.; Li, H.; Yang, J.; Zhong, H.; Zhang, Z. Plasma Neuregulin 4 Levels Are Associated with Metabolic Syndrome in Patients Newly Diagnosed with Type 2 Diabetes Mellitus. Dis. Markers 2018, 2018, 6974191. [Google Scholar] [CrossRef] [Green Version]
- Yan, P.; Xu, Y.; Zhang, Z.; Gao, C.; Zhu, J.; Li, H.; Wan, Q. Decreased plasma neuregulin 4 levels are associated with peripheral neuropathy in Chinese patients with newly diagnosed type 2 diabetes: A cross-sectional study. Cytokine 2019, 113, 356–364. [Google Scholar] [CrossRef]
- Kocak, M.Z.; Aktas, G.; Atak, B.M.; Duman, T.T.; Yis, O.M.; Erkus, E.; Savli, H. Is Neuregulin-4 a predictive marker of microvascular complications in type 2 diabetes mellitus? Eur. J. Clin. Investig. 2020, 50, e13206. [Google Scholar] [CrossRef]
- Li, Y.; Teng, D.; Shi, X.; Qin, G.; Qin, Y.; Quan, H.; Shi, B.; Sun, H.; Ba, J.; Chen, B.; et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: National cross sectional study. BMJ 2020, 369, m997. [Google Scholar] [CrossRef] [PubMed]
- Shoukry, A.; Bdeer, S.e.l.-A.; El-Sokkary, R.H. Urinary monocyte chemoattractant protein-1 and vitamin D-binding protein as biomarkers for early detection of diabetic nephropathy in type 2 diabetes mellitus. Mol. Cell. Biochem. 2015, 408, 25–35. [Google Scholar] [CrossRef]
- Lee, B.W.; Ihm, S.H.; Choi, M.G.; Yoo, H.J. The comparison of cystatin C and creatinine as an accurate serum marker in the prediction of type 2 diabetic nephropathy. Diabetes Res. Clin. Pract. 2007, 78, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zou, H.; Xu, G. Clinical Utility of Serum Cystatin C in Predicting Diabetic Nephropathy Among Patients with Diabetes Mellitus: A Meta-Analysis. Kidney Blood Press Res. 2016, 41, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.F.; Mora, C. Diabetes, inflammation, proinflammatory cytokines, and diabetic nephropathy. Sci. World J. 2006, 6, 908–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, G.; Nakano, K.; Sawada, M.; Uno, K.; Shibayama, Y.; Ienaga, K.; Kondo, M. Possible role of tumor necrosis factor and interleukin-1 in the development of diabetic nephropathy. Kidney Int. 1991, 40, 1007–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, J.F.; Mora, C.; Maca, M.; Garca, J. Inflammatory parameters are independently associated with urinary albumin in type 2 diabetes mellitus. Am. J. Kidney Dis. 2003, 42, 53–61. [Google Scholar] [CrossRef]
- Kalantarinia, K.; Awad, A.S.; Siragy, H.M. Urinary and renal interstitial concentrations of TNF-alpha increase prior to the rise in albuminuria in diabetic rats. Kidney Int. 2003, 64, 1208–1213. [Google Scholar] [CrossRef] [Green Version]
- Uwaezuoke, S.N. The role of novel biomarkers in predicting diabetic nephropathy: A review. Int. J. Nephrol. Renovasc. Dis. 2017, 10, 221–231. [Google Scholar] [CrossRef] [Green Version]
- Mao, S.; Xiang, W.; Huang, S.; Zhang, A. Association between homocysteine status and the risk of nephropathy in type 2 diabetes mellitus. Clin. Chim. Acta 2014, 431, 206–210. [Google Scholar] [CrossRef]
- Ma, L.; Liu, Q.; Jiang, Y.; Zhao, H.; Zhao, T.; Cao, Y.; Li, P.; Niu, W. Genetically elevated circulating homocysteine concentrations increase the risk of diabetic kidney disease in Chinese diabetic patients. J. Cell. Mol. Med. 2019, 23, 2794–2800. [Google Scholar] [CrossRef] [Green Version]
- Tutunchi, H.; Ostadrahimi, A.; Hosseinzadeh-Attar, M.J.; Miryan, M.; Mobasseri, M.; Ebrahimi-Mameghani, M. A systematic review of the association of neuregulin 4, a brown fat-enriched secreted factor, with obesity and related metabolic disturbances. Obes. Rev. 2020, 21, e12952. [Google Scholar] [CrossRef]
- Wang, H.; Cui, K.; Xu, K.; Xu, S. Association between plasma homocysteine and progression of early nephropathy in type 2 diabetic patients. Int. J. Clin. Exp. Med. 2015, 8, 11174–11180. [Google Scholar] [PubMed]
- Cai, C.; Lin, M.; Xu, Y.; Li, X.; Yang, S.; Zhang, H. Association of circulating neuregulin 4 with metabolic syndrome in obese adults: A cross-sectional study. BMC Med. 2016, 14, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, P.; Zhang, Z.; Miao, Y.; Xu, Y.; Zhu, J.; Wan, Q. Changes of circulating neuregulin 4 and its relationship with 25-hydroxy vitamin D and other diabetic vascular complications in patients with diabetic peripheral neuropathy. Diabetol. Metab. Syndr. 2020, 12, 42. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Control (N = 43) | DM (N = 55) | DKD (N = 85) | |
|---|---|---|---|---|
| Gender (%) | Male | 16 (37.21) | 19 (34.55) | 41 (48.24) |
| Female | 27 (62.79) | 36 (65.45) | 44 (51.76) | |
| Age (mean (SD)), y | 45.49 (18.72) | 53.02 (16.09) a | 55.27 (15.41) b | |
| BMI (mean (SD)), kg/m2 | 22.29 (2.89) | 23.72 (4.00) a | 23.71 (3.33) b | |
| UACR (mean (SD)), mg/g | 15.66 (6.83) | 21.54 (5.85) a | 147.75 (147.54) b,c | |
| eGFR (mean (SD)), ml/min | 103.38 (15.30) | 96.75 (17.50) | 85.74 (18.66) b,c | |
| HCY (mean (SD)), μmol/l | 9.46 (2.84) | 14.34 (3.96) a | 20.16 (4.08) b,c | |
| NRG4 (mean (SD)), ng/ml | 1.31 (0.17) | 1.07 (0.16) a | 0.80 (0.14) b,c | |
| HCY-to NRG4 ratio (mean (SD)) | 7.44 (2.99) | 13.61 (3.73) a | 25.98 (7.30) b,c | |
| FBG (mean (SD)), mmol/l | 5.61 (1.08) | 9.73 (4.71) a | 10.03 (4.34) b | |
| HbA1C (mean (SD)) | 5.34 (0.41) | 9.12 (1.63) a | 9.33 (1.72) b | |
| BUN (mean (SD)), mmol/l | 4.33 (0.94) | 5.60 (1.44) a | 6.70 (1.65) b,c | |
| SCR (mean (SD)), μmol/l | 65.70 (12.16) | 70.13 (17.37) | 74.27 (23.14) b | |
| LDL (mean (SD)), mmol/l | 1.89 (0.71) | 2.61 (0.81) a | 2.65 (0.70) b | |
| TCHOL (mean (SD)), mmol/l | 4.14 (0.82) | 4.59 (0.95) a | 4.83 (0.71) b | |
| HDL (mean (SD)), mmol/l | 1.54 (0.58) | 1.11 (0.34) a | 0.99 (0.22) b,c | |
| TG (mean (SD)), mmol/l | 1.41 (0.95) | 1.90 (0.84) | 1.97 (0.96) b | |
| UA (mean (SD)), μmol/l | 298.00 (278.50) | 307.80 (65.66) | 354.66 (90.43) b,c | |
| Course of DM (mean (SD)), month | 48.16 (65.89) | 109.17 (87.31) c | ||
| DR (%) | No | 41 (74.55) | 41 (48.24) c | |
| Yes | 14 (25.45) | 44 (51.76) | ||
| PAD (%) | No | 21 (38.18) | 30 (35.29) | |
| Yes | 34 (61.82) | 55 (64.71) | ||
| DPN (%) | No | 25 (45.45) | 38 (44.71) | |
| Yes | 30 (54.55) | 47 (55.29) | ||
| HBP (%) | No | 36 (65.45) | 41 (48.24) | |
| Yes | 19 (34.55) | 44 (51.76) | ||
| CHD (%) | No | 27 (49.09) | 52 (61.18) | |
| Yes | 28 (50.91) | 33 (38.82) | ||
| Stroke (%) | No | 38 (69.09) | 55 (64.71) | |
| Yes | 17 (30.91) | 30 (35.29) |
| OR | 95% CI | p | ||
|---|---|---|---|---|
| Lower Limit | Upper Limit | |||
| HCY-to-NRG4 ratio | 1.870 | 1.496 | 2.573 | <0.001 * |
| BUN | 1.441 | 0.917 | 2.356 | 0.121 |
| HDL | 1.094 | 0.063 | 17.802 | 0.949 |
| UA | 0.993 | 0.983 | 1.004 | 0.227 |
| DR | 0.432 | 0.391 | 7.510 | 0.323 |
| Course of DM | 1.015 | 1.004 | 1.029 | 0.012 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, S.; Yang, Y.; Zheng, Y.; Xu, J.; Cheng, Y.; Wei, W.; Yu, F.; Li, L.; Li, M.; Wang, M.; et al. Diagnostic Value of the Combined Measurement of Serum HCY and NRG4 in Type 2 Diabetes Mellitus with Early Complicating Diabetic Nephropathy. J. Pers. Med. 2023, 13, 556. https://doi.org/10.3390/jpm13030556
Ding S, Yang Y, Zheng Y, Xu J, Cheng Y, Wei W, Yu F, Li L, Li M, Wang M, et al. Diagnostic Value of the Combined Measurement of Serum HCY and NRG4 in Type 2 Diabetes Mellitus with Early Complicating Diabetic Nephropathy. Journal of Personalized Medicine. 2023; 13(3):556. https://doi.org/10.3390/jpm13030556
Chicago/Turabian StyleDing, Sheng, Yi Yang, Yuming Zheng, Jinling Xu, Yangyang Cheng, Wei Wei, Fuding Yu, Li Li, Menglan Li, Mengjie Wang, and et al. 2023. "Diagnostic Value of the Combined Measurement of Serum HCY and NRG4 in Type 2 Diabetes Mellitus with Early Complicating Diabetic Nephropathy" Journal of Personalized Medicine 13, no. 3: 556. https://doi.org/10.3390/jpm13030556
APA StyleDing, S., Yang, Y., Zheng, Y., Xu, J., Cheng, Y., Wei, W., Yu, F., Li, L., Li, M., Wang, M., Wang, Z., & Xiang, G. (2023). Diagnostic Value of the Combined Measurement of Serum HCY and NRG4 in Type 2 Diabetes Mellitus with Early Complicating Diabetic Nephropathy. Journal of Personalized Medicine, 13(3), 556. https://doi.org/10.3390/jpm13030556