O-Arm- and Guide-Device-Assisted Personalized Percutaneous Kyphoplasty for Thoracolumbar Osteoporotic Vertebral Compression Fractures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
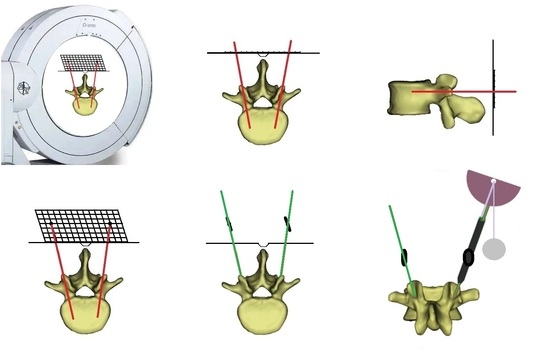
2.2. Guide Device
2.3. Surgical Procedures
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
3.1. General Information
3.2. Perioperative Outcome Comparison
3.3. Clinical and Radiological Outcome Comparison
3.4. Complication Comparison
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Yu, W.; Yin, X.; Cui, L.; Tang, S.; Jiang, N.; Cui, L.; Zhao, N.; Lin, Q.; Chen, L.; et al. Prevalence of Osteoporosis and Fracture in China: The China Osteoporosis Prevalence Study. JAMA Netw. Open 2021, 4, e2121106. [Google Scholar] [CrossRef]
- Li, J.; Yuan, X.; Li, F.; Ding, Y.; Ma, G.; Song, C.; Chen, X.; Wang, E.; Cui, J.; Kong, Q.; et al. A randomized trial comparing the clinical efficacy and safety of a novel steerable percutaneous kyphoplasty with traditional PKP in osteoporotic vertebral fractures. Ann. Transl. Med. 2021, 9, 1024. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Yi, W.; Yang, D. Advances in Vertebral Augmentation Systems for Osteoporotic Vertebral Compression Fractures. Pain Res. Manag. 2020, 2020, 3947368. [Google Scholar] [CrossRef]
- Jin, M.; Ge, M.; Lei, L.; Li, F.; Wu, M.; Zhang, G.; Pei, S.; Zheng, B. Clinical and Radiologic Outcomes of Robot-Assisted Kyphoplasty versus Fluoroscopy-Assisted Kyphoplasty in the Treatment of Osteoporotic Vertebral Compression Fractures: A Retrospective Comparative Study. World Neurosurg. 2022, 158, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Meng, X.; Cao, W.; Zhu, Y. Robot-Assisted Versus Fluoroscopy-Assisted Kyphoplasty in the Treatment of Osteoporotic Vertebral Compression Fracture: A Retrospective Study. Glob. Spine J. 2022, 12, 1151–1157. [Google Scholar] [CrossRef]
- Yu, H.; Luo, G.; Yu, B.; Sun, T.; Tang, Q.; Jia, Y. Robot-Assisted Kyphoplasty Improves Clinical and Radiological Features Better Than Fluoroscopy-Assisted Kyphoplasty in the Treatment of Vertebral Compression Fractures: A Meta-Analysis. Front. Surg. 2022, 9, 955966. [Google Scholar] [CrossRef]
- Qian, J.; Fang, C.; Ge, P.; Zhang, R.J.; Song, P.W.; Xu, P.; Zhang, Y.; Shen, C.L. Efficacy and Safety of Establishing an Optimal Path Through Unilateral Pedicle Under the Assistance of Surgical Robot in Percutaneous Kyphoplasty. Pain Physician 2020, 25, E133–E140. [Google Scholar]
- Wang, B.; Cao, J.; Chang, J.; Yin, G.; Cai, W.; Li, Q.; Huang, Z.; Yu, L.; Cao, X. Effectiveness of Tirobot-assisted vertebroplasty in treating thoracolumbar osteoporotic compression fracture. J. Orthop. Surg. Res. 2021, 16, 65. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, N.; Yu, S.; Zhang, T.; Wang, C.; He, S.; Gu, G. Reduced Radiation Exposure and Puncture Time of Percutaneous Transpedicular Puncture with Real-Time Ultrasound Volume Navigation. World Neurosurg. 2018, 119, e997–e1005. [Google Scholar] [CrossRef]
- He, G.; Yu, Q.; Yang, K.; Gao, Z.; Han, A. Clinical application of percutaneous kyphoplasty under the guidance of DynaCT in the treatment of compression fractures of thoracolumbar. J. Musculoskelet. Neuronal. Interact. 2019, 19, 229–233. [Google Scholar]
- Jia, Y.; Peng, Z.; Li, J.; Qin, Y.; Wang, G. Unilateral Percutaneous Kyphoplasty with O-Arm Navigation for the Treatment of Kümmell’s Disease. J. Pain Res. 2022, 15, 257–266. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, H.; He, F.; Chen, A.; Yang, H.; Pi, B. Safety and efficacy of percutaneous kyphoplasty assisted with O-arm navigation for the treatment of osteoporotic vertebral compression fractures at T6 to T9 vertebrae. Int. Orthop. 2020, 44, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.Z.; Wang, Y.M.; Liang, X.; Ze, X.; Liu, H.; Chen, K.W.; Zhu, X.Y.; Sun, Z.Y.; Qian, Z.L. Minimally Invasive Pedicle Screw Fixation Combined with Percutaneous Kyphoplasty under O-Arm Navigation for the Treatment of Metastatic Spinal Tumors with Posterior Wall Destruction. Orthop. Surg. 2020, 12, 1131–1139. [Google Scholar] [CrossRef]
- Gu, H.; Zhao, Y.; Xie, Y.; Wei, Y.; Li, L.; Meng, D.; Yu, H. Combined Application of Grid Body Surface Locator and Preemptive Analgesia in Daytime Vertebroplasty. Comput. Math. Methods Med. 2022, 2022, 2651062. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Gu, H.; Yongcun, W.; Zhao, Y.; Xiang, L.; Meng, D.; Wang, A.; Yu, H. A Comparison between Accurate Unilateral Puncture Paths Planned by Preoperative and Conventional Unilateral Puncture Techniques in Percutaneous Vertebroplasty. Comput. Math. Methods Med. 2022, 2022, 6762530. [Google Scholar] [CrossRef]
- Lin, G.X.; Chen, C.M.; Rui, G.; Hu, B.S. Research relating to three-dimensional (3D) printing in spine surgery: A bibliometric analysis. Eur. Spine J. 2022, 32, 395–407. [Google Scholar] [CrossRef]
- Hu, P.L.; Lin, J.S.; Meng, H.; Su, N.; Yang, Y.; Fei, Q. A novel “three-dimensional-printed individual guide template-assisted percutaneous vertebroplasty” for osteoporotic vertebral compression fracture: A prospective, controlled study. J. Orthop. Surg. Res. 2021, 16, 326. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Lin, J.; Xu, J.; Meng, H.; Su, N.; Yang, Y.; Fei, Q. Three-Dimensional Printing Guide Template Assisted Percutaneous Vertebroplasty (PVP). J. Vis. Exp. 2019, 152, e60010. [Google Scholar]
- Li, J.; Lin, J.; Yang, Y.; Xu, J.; Fei, Q. 3-Dimensional printing guide template assisted percutaneous vertebroplasty: Technical note. J. Clin. Neurosci. 2018, 52, 159–164. [Google Scholar] [CrossRef]
- Li, J.; Lin, J.; Xu, J.; Meng, H.; Su, N.; Fan, Z.; Li, J.; Yang, Y.; Li, D.; Wang, B.; et al. A Novel Approach for Percutaneous Vertebroplasty Based on Preoperative Computed Tomography-Based Three-Dimensional Model Design. World Neurosurg. 2017, 105, 20–26. [Google Scholar] [CrossRef]
- Wang, H.; Yu, H.; Zhu, Y.; Gu, H.; Zheng, B.; Zhao, Y.; Han, W.; Xiang, L. Unilateral Percutaneous Kyphoplasty Using a Novel Guide Device for Thoracolumbar Osteoporotic Vertebral Fracture. Orthop. Surg. 2023. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Kunisada, T.; Takeda, K.; Hasei, J.; Nakata, E.; Nakahara, R.; Yoshida, A.; Ozaki, T. Intraoperative O-arm-navigated resection in musculoskeletal tumors. J. Orthop. Sci. 2018, 23, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Huang, X.; Luo, L.; Liu, H.; Wu, H.; Tan, Y.; Li, C.; Tang, Y.; Zhou, Y. Radiation Dose Reduction and Surgical Efficiency Improvement in Endoscopic Transforaminal Lumbar Interbody Fusion Assisted by Intraoperative O-arm Navigation: A Retrospective Observational Study. Neurospine 2022, 19, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wang, W.; Chen, S.; Wu, K.; Wang, H. O-arm navigation versus C-arm guidance for pedicle screw placement in spine surgery: A systematic review and meta-analysis. Int. Orthop. 2020, 44, 919–926. [Google Scholar] [CrossRef]
- Croci, D.M.; Nguyen, S.; Streitmatter, S.W.; Sherrod, B.A.; Hardy, J.; Cole, K.L.; Gamblin, A.S.; Bisson, E.F.; Mazur, M.D.; Dailey, A.T. O-Arm Accuracy and Radiation Exposure in Adult Deformity Surgery. World Neurosurg. 2022, 171, e440–e446. [Google Scholar] [CrossRef]
- Jing, L.; Sun, Z.; Zhang, P.; Wang, J.; Wang, G. Accuracy of Screw Placement and Clinical Outcomes After O-Arm-Navigated Occipitocervical Fusion. World Neurosurg. 2018, 117, e653–e659. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.; Chen, H.; Zhang, K.; Lu, J.; Mao, H.; Yang, H. Comparison between free-hand and O-arm-based navigated posterior lumbar interbody fusion in elderly cohorts with three-level lumbar degenerative disease. Int. Orthop. 2019, 43, 351–357. [Google Scholar] [CrossRef]
- Lee, M.J.; Dumonski, M.; Cahill, P.; Stanley, T.; Park, D.; Singh, K. Percutaneous treatment of vertebral compression fractures: A meta-analysis of complications. Spine 2009, 34, 1228–1232. [Google Scholar] [CrossRef]
- Ma, X.; Sun, H.; Liu, S.; Sang, L.; Wang, K.; Dong, Y.; Qi, X. Cement Leakage in Vertebral Compression Fractures Between Unilateral and Bilateral Percutaneous Vertebral Augmentation: A Meta-Analysis. Turk. Neurosurg. 2022, 32, 883–892. [Google Scholar] [CrossRef]
- Zhan, Y.; Jiang, J.; Liao, H.; Tan, H.; Yang, K. Risk Factors for Cement Leakage After Vertebroplasty or Kyphoplasty: A Meta-Analysis of Published Evidence. World Neurosurg. 2017, 101, 633–642. [Google Scholar] [CrossRef]
- Kanat, A.; Tsianaka, E.; Gasenzer, E.R.; Drosos, E. Some Interesting Points of Competition of X-Ray using during the Greco-Ottoman War in 1897 and Development of Neurosurgical Radiology: A Reminiscence. Turk. Neurosurg. 2022, 32, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, B.; Kanat, A.; Erturk, C.; Batcik, O.E.; Balik, M.S.; Yazar, U.; Celiker, F.B.; Metin, Y.; Inecikli, M.F.; Guvercin, A.R. Restoration of Anterior Vertebral Height by Short-Segment Pedicle Screw Fixation with Screwing of Fractured Vertebra for the Treatment of Unstable Thoracolumbar Fractures. World Neurosurg. 2017, 99, 409–417. [Google Scholar] [CrossRef] [PubMed]




| Characteristic | TF Group (n = 22) | O-GD Group (n = 16) | p | |
|---|---|---|---|---|
| Male/female | 7/15 | 4/12 | 0.924 | |
| Mean age (years) | 69.9 ± 7.8 | 71.6 ± 8.3 | 0.520 | |
| BMI (kg/m2) | 22.3 ± 2.7 | 23.9 ± 2.1 | 0.054 | |
| BMD | −3.0 ± 0.5 | −3.2 ± 0.6 | 0.377 | |
| Mean follow-up duration (months) | 15.5 ± 3.2 | 15.6 ± 5.1 | 0.991 | |
| Fractured vertebra | ||||
| T11 | 3 | 3 | 0.623 | |
| T12 | 6 | 7 | ||
| L1 | 9 | 4 | ||
| L2 | 4 | 2 | ||
| Characteristic | TF Group (n = 22) | O-GD Group (n = 16) | p |
|---|---|---|---|
| Operation time (mins) | 57.2 ± 9.7 | 38.3 ± 12.2 | <0.001 |
| Intraoperative blood loss (mL) | 9.1 ± 3.3 | 6.9 ± 2.5 | 0.031 |
| Intraoperative fluoroscopy exposures | 46.7 ± 7.2 | 28.9 ± 4.5 | <0.001 |
| Volume of injected cement (mL) | 6.8 ± 1.3 | 6.7 ± 1.7 | 0.854 |
| Postoperative hospital stay (days) | 1.5 ± 1.4 | 1.1 ± 0.5 | 0.326 |
| Hospitalization cost (×104 CNY) | 3.4 ± 0.2 | 3.5 ± 0.2 | 0.107 |
| Characteristic | TF Group (n = 22) | O-GD Group (n = 16) | p | |
|---|---|---|---|---|
| VAS score | ||||
| Preoperative | 7.2 ± 0.9 | 7.4 ± 0.6 | 0.416 | |
| 1 day postoperative | 3.5 ± 0.8 | 3.1 ± 0.8 | 0.139 | |
| 1 month postoperative | 2.9 ± 0.9 | 2.3 ± 0.9 | 0.052 | |
| 12 months postoperative | 1.7 ± 0.6 | 1.4 ± 0.5 | 0.108 | |
| ODI | ||||
| Preoperative | 76.1 ± 10.4 | 78.7 ± 3.9 | 0.303 | |
| 1 day postoperative | 37.5 ± 6.1 | 39.5 ± 4.5 | 0.285 | |
| 1 month postoperative | 28.4 ± 3.7 | 28.0 ± 3.9 | 0.772 | |
| 12 months postoperative | 15.8 ± 4.4 | 16.4 ± 3.1 | 0.670 | |
| AVH (mm) | ||||
| Preoperative | 22.3 ± 5.5 | 21.0 ± 5.3 | 0.452 | |
| 1 day postoperative | 27.8 ± 4.5 | 26.8 ± 5.1 | 0.557 | |
| 12 months postoperative | 26.7 ± 4.4 | 25.3 ± 4.4 | 0.341 | |
| LKA (°) | ||||
| Preoperative | 11.5 ± 4.9 | 11.5 ± 6.1 | 0.988 | |
| 1 day postoperative | 7.6 ± 3.7 | 7.1 ± 4.9 | 0.736 | |
| 12 months postoperative | 8.8 ± 3.9 | 8.1 ± 5.0 | 0.652 | |
| Characteristic | TF Group (n = 22) | O-GD Group (n = 16) | p |
|---|---|---|---|
| Refracture (n) | 0 | 1 (6.3%) | 0.871 |
| Cement leakage (n) | 9 (40.9%) | 3 (18.8%) | 0.272 |
| Neurological deficit (n) | 0 | 0 | - |
| Pulmonary embolism (n) | 0 | 0 | - |
| Infection (n) | 0 | 0 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zheng, B.; Gu, H.; Zhao, Y.; Liu, D.; Yu, H.; Xiang, L. O-Arm- and Guide-Device-Assisted Personalized Percutaneous Kyphoplasty for Thoracolumbar Osteoporotic Vertebral Compression Fractures. J. Pers. Med. 2023, 13, 595. https://doi.org/10.3390/jpm13040595
Wang H, Zheng B, Gu H, Zhao Y, Liu D, Yu H, Xiang L. O-Arm- and Guide-Device-Assisted Personalized Percutaneous Kyphoplasty for Thoracolumbar Osteoporotic Vertebral Compression Fractures. Journal of Personalized Medicine. 2023; 13(4):595. https://doi.org/10.3390/jpm13040595
Chicago/Turabian StyleWang, Hongwei, Bin Zheng, Hongwen Gu, Yuanhang Zhao, Da Liu, Hailong Yu, and Liangbi Xiang. 2023. "O-Arm- and Guide-Device-Assisted Personalized Percutaneous Kyphoplasty for Thoracolumbar Osteoporotic Vertebral Compression Fractures" Journal of Personalized Medicine 13, no. 4: 595. https://doi.org/10.3390/jpm13040595
APA StyleWang, H., Zheng, B., Gu, H., Zhao, Y., Liu, D., Yu, H., & Xiang, L. (2023). O-Arm- and Guide-Device-Assisted Personalized Percutaneous Kyphoplasty for Thoracolumbar Osteoporotic Vertebral Compression Fractures. Journal of Personalized Medicine, 13(4), 595. https://doi.org/10.3390/jpm13040595