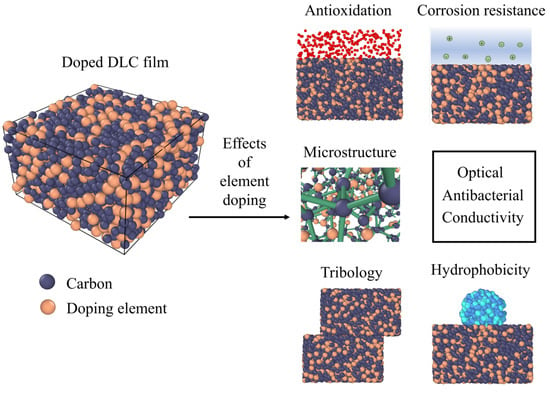
Effects of Element Doping on the Structure and Properties of Diamond-like Carbon Films: A Review
Abstract
:1. Introduction
2. Microstructure
2.1. DLC Film Structure
2.2. Roughness
3. Mechanical Properties
3.1. Residual Stress
3.2. Hardness and Elastic Modulus
4. Tribological Properties
4.1. Carbide-Forming Element Doping
4.2. Non-Carbide-Forming Element Doping
4.3. Multielement Doping
5. Other Properties
5.1. Corrosion Resistance
5.2. Hydrophobic Properties
5.3. Antioxidation Properties
5.4. Antibacterial Properties
5.5. Biotribological Properties
5.6. Electrical Conductivity, Optical, and Triboelectrification Properties
6. Conclusions and Prospect
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, J.; Zhu, L.; Liu, G.; Song, B. Study on the frictional wear performance of DLC-PFPE solid-liquid composite lubrication system. Mater. Direct 2020, 34, 567–571. [Google Scholar]
- Dekempeneer, E.; Van Acker, K.; Vercammen, K.; Meneve, J.; Neerinck, D.; Eufinger, S.; Smeets, J. Abrasion resistant low friction diamond-like multilayers. Surf. Coat. Technol. 2001, 142, 669–673. [Google Scholar] [CrossRef]
- Erdemir, A.; Wryilmaz, O. Achieving superlubricity in DLC films by controlling bulk, surface, and tribochemistry. Friction 2014, 2, 140–155. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, B.; Cao, Z.; Shi, P.; Zhou, N.; Zhang, B.; Qian, L. Probing superlubricity stability of hydrogenated diamond-like carbon film by varying sliding velocity. Appl. Surf. Sci. 2018, 439, 976–982. [Google Scholar] [CrossRef]
- Aisenberg, S.; Chabot, R. Ion-beam deposition of thin films of diamondlike carbon. J. Appl. Phys. 1971, 42, 2953–2958. [Google Scholar] [CrossRef]
- Stock, F.; Antoni, F.; Aubel, D.; Hajjar-Garreau, S.; Muller, D. Pure carbon conductive transparent electrodes synthetized by a full laser deposition and annealing process. Appl. Surf. Sci. 2020, 505, 144505. [Google Scholar] [CrossRef]
- Gao, K.; Wei, X.; Liu, G.; Zhang, B.; Zhang, J. Electrodeposition and biocompatibility of palladium and phosphorus doped amorphous hydrogenated carbon films. Chem. Phys. 2020, 537, 110857. [Google Scholar] [CrossRef]
- Jurewicz, A.J.G.; Rieck, K.D.; Hervig, R.; Burnett, D.S.; Wadhwa, M.; Olinger, C.T.; Williams, P. Magnesium isotopes of the bulk solar wind from Genesis diamond-like carbon films. Meteorit. Planet. Sci. 2020, 55, 352–375. [Google Scholar] [CrossRef]
- Sunthornpan, N.; Watanabe, S.; Moolsradoo, N. Elements-added diamond-like carbon film for biomedical applications. Adv. Mater. Sci. Eng. 2019, 2019, 6812092. [Google Scholar] [CrossRef]
- Luo, J.K.; Fu, Y.Q.; Le, H.R.; Williams, J.A.; Spearing, S.M.; Milne, W.I. Diamond and diamond-like carbon MEMS. J. Micromech. Microeng. 2007, 17, S147. [Google Scholar] [CrossRef]
- Grigoriev, S.; Volosova, M.; Fyodorov, S.; Lyakhovetskiy, M.; Seleznev, A. DLC-coating application to improve the durability of ceramic tools. J. Mater. Eng. Perform. 2019, 28, 4415–4426. [Google Scholar] [CrossRef]
- REJOWSKI, E.D.; Mordente, P.; PILLIS, M.F.; Casserly, T. Application of DLC coating in cylinder liners for friction reduction. SAE Int. 2014. [CrossRef]
- Kano, M. Overview of DLC-coated engine components. Coat. Technol. Veh. Appl. 2015, 37–62. [Google Scholar] [CrossRef]
- Marian, M.; Weikert, T.; Tremmel, S. On friction reduction by surface modifications in the TEHL cam/tappet-contact-experimental and numerical studies. Coatings 2019, 9, 843. [Google Scholar] [CrossRef]
- Kröner, J.; Tremmel, S.; Kursawe, S.; Musayev, Y.; Hosenfeldt, T.; Wartzack, S. GreenBearings–Friction Behaviour of DLC-Coated Dry Running Deep Groove Ball Bearings. In Applied Mechanics and Materials; Trans Tech Publications Ltd.: Zurich, Switzerland, 2015; Volume 805, pp. 147–153. [Google Scholar]
- Zhang, S.; Xie, H.; Zeng, X.; Hing, P. Residual stress characterization of diamond-like carbon coatings by an X-ray diffraction method. Surf. Coat. Technol. 1999, 122, 219–224. [Google Scholar] [CrossRef]
- Nakazawa, H.; Yamagata, Y.; Suemitsu, M.; Mashita, M. Thermal effects on structural properties of diamond-like carbon films prepared by pulsed laser deposition. Thin Solid Films 2004, 467, 98–103. [Google Scholar] [CrossRef]
- Zhang, Y.; Polychronopoulou, K.; Humood, M.; Polycarpou, A.A. High temperature nanotribology of ultra-thin hydrogenated amorphous carbon coatings. Carbon 2017, 123, 112–121. [Google Scholar] [CrossRef]
- Shao, W.; Zhou, Y.; Shi, Z.; Rao, L.; Hu, T.; Xing, X.; Yang, Q. Effects of carbide forming elements Me on residual stress and mechanical properties of DLC films by molecular dynamics simulation. Mater. Today Commun. 2020, 23, 100946. [Google Scholar] [CrossRef]
- Cao, H.; Ye, X.; Li, H.; Qi, F.; Wang, Q.; Ouyang, X.; Liao, B. Microstructure, mechanical and tribological properties of multilayer Ti-DLC thick films on Al alloys by filtered cathodic vacuum arc technology. Mater. Des. 2021, 198, 109320. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, L.; Shao, W.; Chen, Z.; Wang, S.; Xing, X.; Yang, Q. Mechanical and tribological behaviors of Ti-DLC films deposited on 304 stainless steel: Exploration with Ti doping from micro to macro. Diam. Relat. Mater. 2020, 107, 107870. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, Z.; Piliptsou, D.G.; Yu, S.; Wang, Z.; Rogachev, A.V.; Balmakou, A. Structure and optical properties of Cu-DLC composite films deposited by cathode arc with double-excitation source. Diam. Relat. Mater. 2016, 69, 191–197. [Google Scholar] [CrossRef]
- Baba, K.; Hatada, R.; Flege, S.; Ensinger, W.; Shibata, Y.; Nakashima, J.; Morimura, T. Preparation and antibacterial properties of Ag-containing diamond-like carbon films prepared by a combination of magnetron sputtering and plasma source ion implantation. Vacuum 2013, 89, 179–184. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Wang, X. Structure characterization and antibacterial properties of Ag-DLC films fabricated by dual-targets HiPIMS. Surf. Coat. Technol. 2021, 410, 126967. [Google Scholar] [CrossRef]
- Wang, A.Y.; Lee, K.R.; Ahn, J.P.; Han, J.H. Structure and mechanical properties of W incorporated diamond-like carbon films prepared by a hybrid ion beam deposition technique. Carbon 2006, 44, 1826–1832. [Google Scholar] [CrossRef]
- Dai, W.; Wang, A. Synthesis, characterization and properties of the DLC films with low Cr concentration doping by a hybrid linear ion beam system. Surf. Coat. Technol. 2011, 205, 2882–2886. [Google Scholar] [CrossRef]
- Qiang, L.; Zhang, B.; Zhou, Y.; Zhang, J. Improving the internal stress and wear resistance of DLC film by low content Ti doping. Solid State Sci. 2013, 20, 17–22. [Google Scholar] [CrossRef]
- Choi, H.W.; Choi, J.H.; Lee, K.R.; Ahn, J.P.; Oh, K.H. Structure and mechanical properties of Ag-incorporated DLC films prepared by a hybrid ion beam deposition system. Thin Solid Films 2007, 516, 248–251. [Google Scholar] [CrossRef]
- Bouabibsa, I.; Lamri, S.; Sanchette, F. Structure, mechanical and tribological properties of Me-doped diamond-like carbon (DLC)(Me= Al, Ti, or Nb) hydrogenated amorphous carbon coatings. Coatings 2018, 8, 370. [Google Scholar] [CrossRef]
- Tang, X.S.; Wang, H.J.; Feng, L.; Shao, L.X.; Zou, C.W. Mo doped DLC nanocomposite coatings with improved mechanical and blood compatibility properties. Appl. Surf. Sci. 2014, 311, 758–762. [Google Scholar] [CrossRef]
- Santiago, J.A.; Fernández-Martínez, I.; Sánchez-López, J.C.; Rojas, T.C.; Wennberg, A.; Bellido-González, V.; González-Arrabal, R. Tribomechanical properties of hard Cr-doped DLC coatings deposited by low-frequency HiPIMS. Surf. Coat. Technol. 2020, 382, 124899. [Google Scholar] [CrossRef]
- Zhao, F.; Li, H.; Ji, L.; Wang, Y.; Liu, X.; Zhou, H.; Chen, J. Effect of microstructural evolution on mechanical and tribological properties of Ti-doped DLC films: How was an ultralow friction obtained? J. Vac. Sci. Technol. A Vac. Surf. Film. 2016, 34, 031504. [Google Scholar] [CrossRef]
- Dai, W.; Wang, A.; Wang, Q. Microstructure and mechanical property of diamond-like carbon films with ductile copper incorporation. Surf. Coat. Technol. 2015, 272, 33–38. [Google Scholar] [CrossRef]
- Dai, W.; Ke, P.; Wang, A. Microstructure and property evolution of Cr-DLC films with different Cr content deposited by a hybrid beam technique. Vacuum 2011, 85, 792–797. [Google Scholar] [CrossRef]
- Dai, W.; Wang, A. Deposition and properties of Al-containing diamond-like carbon films by a hybrid ion beam sources. J. Alloys Compd. 2011, 509, 4626–4631. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, L.; Xue, Q. The structure and tribological properties of aluminum/carbon nanocomposite thin films synthesized by reactive magnetron sputtering. Surf. Interface Anal. 2011, 43, 1057–1063. [Google Scholar] [CrossRef]
- Manninen, N.K.; Ribeiro, F.; Escudeiro, A.; Polcar, T.; Carvalho, S.; Cavaleiro, A. Influence of Ag content on mechanical and tribological behavior of DLC coatings. Surf. Coat. Technol. 2013, 232, 440–446. [Google Scholar] [CrossRef]
- Ding, J.C.; Dai, W.; Zhang, T.F.; Zhao, P.; Yun, J.M.; Kim, K.H.; Wang, Q.M. Microstructure and properties of Nb-doped diamond-like carbon films deposited by high power impulse magnetron sputtering. Thin Solid Film. 2018, 663, 159–167. [Google Scholar] [CrossRef]
- Zhang, K.; Wen, M.; Cheng, G.; Li, X.; Meng, Q.N.; Lian, J.S.; Zheng, W.T. Reactive magnetron sputtering deposition and characterization of niobium carbide films. Vacuum 2014, 99, 233–241. [Google Scholar] [CrossRef]
- Grein, M.; Gerstenberg, J.; von der Heide, C.; Bandorf, R.; Bräuer, G.; Dietzel, A. Niobium-containing DLC coatings on various substrates for strain gauges. Coatings 2019, 9, 417. [Google Scholar] [CrossRef]
- Ding, J.C.; Mei, H.; Jeong, S.; Zheng, J.; Wang, Q.M.; Kim, K.H. Effect of bias voltage on the microstructure and properties of Nb-DLC films prepared by a hybrid sputtering system. J. Alloys Compd. 2021, 861, 158505. [Google Scholar] [CrossRef]
- Khamseh, S.; Alibakhshi, E.; Ramezanzadeh, B.; Sari, M.G. A tailored pulsed substrate bias voltage deposited (aC: Nb) thin-film coating on GTD-450 stainless steel: Enhancing mechanical and corrosion protection characteristics. Chem. Eng. J. 2021, 404, 126490. [Google Scholar] [CrossRef]
- Bharathy, P.V.; Yang, Q.; Kiran, M.S.R.N.; Rha, J.; Nataraj, D.; Mangalaraj, D. Reactive biased target ion beam deposited W–DLC nanocomposite thin films—Microstructure and its mechanical properties. Diam. Relat. Mater. 2012, 23, 34–43. [Google Scholar] [CrossRef]
- Cao, L.; Liu, J.; Wan, Y.; Pu, J. Corrosion and tribocorrosion behavior of W doped DLC coating in artificial seawater. Diam. Relat. Mater. 2020, 109, 108019. [Google Scholar] [CrossRef]
- Baba, K.; Hatada, R.; Tanaka, Y. Preparation and properties of W-containing diamond-like carbon films by magnetron plasma source ion implantation. Surf. Coat. Technol. 2007, 201, 8362–8365. [Google Scholar] [CrossRef]
- Ji, L.; Li, H.; Zhao, F.; Chen, J.; Zhou, H. Microstructure and mechanical properties of Mo/DLC nanocomposite films. Diam. Relat. Mater. 2008, 17, 1949–1954. [Google Scholar] [CrossRef]
- Su, Y.; Cai, L.; Huang, W.; Zhang, T.; Yu, W.; Zhang, P.; Gong, X. Improvement the tribological properties of diamond-like carbon film via Mo doping in diesel condition. Vacuum 2022, 198, 110920. [Google Scholar] [CrossRef]
- Wang, L.L.; Wang, R.Y.; Yan, S.J.; Zhang, R.; Yang, B.; Zhang, Z.D.; Fu, D.J. Structure and properties of Mo-containing diamond-like carbon films produced by ion source assisted cathodic arc ion-plating. Appl. Surf. Sci. 2013, 286, 109–114. [Google Scholar] [CrossRef]
- Ji, L.; Li, H.; Zhao, F.; Quan, W.; Chen, J.; Zhou, H. Atomic oxygen resistant behaviors of Mo/diamond-like carbon nanocomposite lubricating films. Appl. Surf. Sci. 2009, 255, 4180–4184. [Google Scholar] [CrossRef]
- Weicheng, K.; Zhou, Y.; Jun, H. Electrochemical performance and corrosion mechanism of Cr–DLC coating on nitrided Ti6Al4V alloy by magnetron sputtering. Diam. Relat. Mater. 2021, 116, 108398. [Google Scholar] [CrossRef]
- Dai, W.; Zheng, H.; Wu, G.; Wang, A. Effect of bias voltage on growth property of Cr-DLC film prepared by linear ion beam deposition technique. Vacuum 2010, 85, 231–235. [Google Scholar] [CrossRef]
- Zou, C.W.; Wang, H.J.; Feng, L.; Xue, S.W. Effects of Cr concentrations on the microstructure, hardness, and temperature-dependent tribological properties of Cr-DLC coatings. Appl. Surf. Sci. 2013, 286, 137–141. [Google Scholar] [CrossRef]
- Písařík, P.; Jelínek, M.; Kocourek, T.; Zezulová, M.; Remsa, J.; Jurek, K. Chromium-doped diamond-like carbon films deposited by dual-pulsed laser deposition. Appl. Phys. A 2014, 117, 83–88. [Google Scholar] [CrossRef]
- Gayathri, S.; Kumar, N.; Krishnan, R.; Ravindran, T.R.; Dash, S.; Tyagi, A.K.; Sridharan, M. Influence of Cr content on the micro-structural and tribological properties of PLD grown nanocomposite DLC-Cr thin films. Mater. Chem. Phys. 2015, 167, 194–200. [Google Scholar] [CrossRef]
- Shaoxian, Z.; Siming, R.; Jibin, P. Tribological Behavior of Cr Doped Diamond-like Carbon Coating in Engine Oil. Chin. J. Mater. Res. 2017, 31, 18–26. [Google Scholar]
- Dai, W.; Ke, P.; Moon, M.W.; Lee, K.R.; Wang, A. Investigation of the microstructure, mechanical properties and tribological behaviors of Ti-containing diamond-like carbon films fabricated by a hybrid ion beam method. Thin Solid Film. 2012, 520, 6057–6063. [Google Scholar] [CrossRef]
- Zhang, M.; Xie, T.; Qian, X.; Zhu, Y.; Liu, X. Mechanical properties and biocompatibility of Ti-doped diamond-like carbon films. ACS Omega 2020, 5, 22772–22777. [Google Scholar] [CrossRef]
- Bai, W.Q.; Li, L.L.; Wang, X.L.; He, F.F.; Liu, D.G.; Jin, G.; Tu, J.P. Effects of Ti content on microstructure, mechanical and tribological properties of Ti-doped amorphous carbon multilayer films. Surf. Coat. Technol. 2015, 266, 70–78. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, M.; Yang, Y.; Zhang, Y.; Yan, F.; Li, H. Excellent mechanical, tribological and anti-corrosive performance of novel Ti-DLC nanocomposite thin films prepared via magnetron sputtering method. Carbon 2019, 151, 136–147. [Google Scholar] [CrossRef]
- Cai, J.B.; Wang, X.L.; Bai, W.Q.; Wang, D.H.; Gu, C.D.; Tu, J.P. Microstructure, mechanical and tribological properties of aC/aC: Ti nanomultilayer film. Surf. Coat. Technol. 2013, 232, 403–411. [Google Scholar] [CrossRef]
- Ma, G.; Gong, S.; Lin, G.; Zhang, L.; Sun, G. A study of structure and properties of Ti-doped DLC film by reactive magnetron sputtering with ion implantation. Appl. Surf. Sci. 2012, 258, 3045–3050. [Google Scholar] [CrossRef]
- Bootkul, D.; Saenphinit, N.; Supsermpol, B.; Aramwit, C.; Intarasiri, S. Synthesis of Ti-doped DLC film on SS304 steels by Filtered Cathodic Vacuum Arc (FCVA) technique for tribological improvement. Appl. Surf. Sci. 2014, 310, 293–299. [Google Scholar] [CrossRef]
- Feng, X.; Xia, Y. Tribological properties of Ti-doped DLC coatings under ionic liquids lubricated conditions. Appl. Surf. Sci. 2012, 258, 2433–2438. [Google Scholar] [CrossRef]
- Gulbiński, W.; Mathur, S.; Shen, H.; Suszko, T.; Gilewicz, A.; Warcholiński, B. Evaluation of phase, composition, microstructure and properties in TiC/aC: H thin films deposited by magnetron sputtering. Appl. Surf. Sci. 2005, 239, 302–310. [Google Scholar] [CrossRef]
- Chen, C.; Tang, W.; Li, X.; Wang, W.; Xu, C. Structure and cutting performance of Ti-DLC films prepared by reactive magnetron sputtering. Diam. Relat. Mater. 2020, 104, 107735. [Google Scholar] [CrossRef]
- Basman, N.; Uzun, R.; Gocer, E.; Bacaksiz, E.; Kolemen, U. Electrodeposition of Si–DLC nanocomposite film and its electronic application. Microsyst. Technol. 2018, 24, 2287–2294. [Google Scholar] [CrossRef]
- Zhao, J.F.; Lemoine, P.; Liu, Z.H.; Quinn, J.P.; McLaughlin, J.A. The effects of Si incorporation on the microstructure and nanomechanical properties of DLC thin films. J. Phys. Condens. Matter 2000, 12, 9201. [Google Scholar] [CrossRef]
- Iseki, T.; Mori, H.; Hasegawa, H.; Tachikawa, H.; Nakanishi, K. Structural analysis of Si-containing diamond-like carbon. Diam. Relat. Mater. 2006, 15, 1004–1010. [Google Scholar] [CrossRef]
- Yin, Y.; Yang, L.; Liu, M.; Chen, D.; Wang, Y.; Zhou, Y. Effect of Si content on the mechanical properties of diamond-like films on AZ31 magnesium alloy. J. Liaoning Univ. Sci. Technol. 2020, 43, 251–258. [Google Scholar]
- Milewski, K.; Madej, M. Structure and mechanical properties of diamond-like carbon (DLC) coatings doped with silicon. Metalurgija 2020, 59, 481–484. [Google Scholar]
- Kim, J.I.; Jang, Y.J.; Kim, J.; Kim, J. Effects of silicon doping on low-friction and high-hardness diamond-like carbon coating via filtered cathodic vacuum arc deposition. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Lubwama, M.; Corcoran, B.; Sayers, K.; Kirabira, J.B.; Sebbit, A.; McDonnell, K.A.; Dowling, D. Adhesion and composite micro-hardness of DLC and Si-DLC films deposited on nitrile rubber. Surf. Coat. Technol. 2012, 206, 4881–4886. [Google Scholar] [CrossRef]
- Yu, W.; Wang, J.; Huang, W.; Cui, L.; Wang, L. Improving high temperature tribological performances of Si doped diamond-like carbon by using W interlayer. Tribol. Int. 2020, 146, 106241. [Google Scholar] [CrossRef]
- Huang, L.; Jiang, H.; Zhang, J.; Zhang, Z.; Zhang, P. Synthesis of copper nanoparticles containing diamond-like carbon films by electrochemical method. Electrochem. Commun. 2006, 8, 262–266. [Google Scholar] [CrossRef]
- Gong, Y.L.; Jing, P.P.; Zhou, Y.J.; Ma, D.L.; Deng, Q.Y.; Shen, R.; Leng, Y.X. Formation of rod-shaped wear debris and the graphitization tendency of Cu-doped hydrogenated diamond-like carbon films. Diam. Relat. Mater. 2020, 102, 107654. [Google Scholar] [CrossRef]
- Khan, M.I.; Adil, F.; Majeed, S.; Farooq, W.A.; Hasan, M.S.; Jabeen, R.; Iqbal, M. Structural, morphological, electrical and optical properties of Cu doped DLC thin films. Mater. Res. Express 2019, 6, 126420. [Google Scholar] [CrossRef]
- Guo, P.; Li, X.; Sun, L.; Chen, R.; Ke, P.; Wang, A. Stress reduction mechanism of diamond-like carbon films incorporated with different Cu contents. Thin Solid Film. 2017, 640, 45–51. [Google Scholar] [CrossRef]
- Khamseh, S.; Alibakhshi, E.; Mahdavian, M.; Saeb, M.R.; Vahabi, H.; Kokanyan, N.; Laheurte, P. Magnetron-sputtered copper/diamond-like carbon composite thin films with super anti-corrosion properties. Surf. Coat. Technol. 2018, 333, 148–157. [Google Scholar] [CrossRef]
- Dwivedi, N.; Kumar, S.; Malik, H.K.; Sreekumar, C.; Dayal, S.; Rauthan, C.M.S.; Panwar, O.S. Investigation of properties of Cu containing DLC films produced by PECVD process. J. Phys. Chem. Solids 2012, 73, 308–316. [Google Scholar] [CrossRef]
- Guo, P.; Sun, L.; Li, X.; Xu, S.; Ke, P.; Wang, A. Structural properties and surface wettability of Cu-containing diamond-like carbon films prepared by a hybrid linear ion beam deposition technique. Thin Solid Films 2015, 584, 289–293. [Google Scholar] [CrossRef]
- Yetim, A.F.; Kovacı, H.; Kasapoğlu, A.E.; Bozkurt, Y.B.; Çelik, A. Influences of Ti, Al and V metal doping on the structural, mechanical and tribological properties of DLC films. Diam. Relat. Mater. 2021, 120, 108639. [Google Scholar] [CrossRef]
- Pu, J.; Zhang, G.; Wan, S.; Zhang, R. Synthesis and characterization of low-friction Al-DLC films with high hardness and low stress. J. Compos. Mater. 2015, 49, 199–207. [Google Scholar] [CrossRef]
- Dai, W.; Ke, P.; Wang, A. Influence of bias voltage on microstructure and properties of Al-containing diamond-like carbon films deposited by a hybrid ion beam system. Surf. Coat. Technol. 2013, 229, 217–221. [Google Scholar] [CrossRef]
- Ding, J.C.; Chen, M.; Mei, H.; Jeong, S.; Zheng, J.; Yang, Y.; Kim, K.H. Microstructure, mechanical, and wettability properties of Al-doped diamond-like films deposited using a hybrid deposition technique: Bias voltage effects. Diam. Relat. Mater. 2022, 123, 108861. [Google Scholar] [CrossRef]
- Xu, W.; Zhou, K.; Lin, S.; Dai, M.; Shi, Q.; Wei, C. Structural properties of hydrogenated Al-doped diamond-like carbon films fabricated by a hybrid plasma system. Diam. Relat. Mater. 2018, 87, 177–185. [Google Scholar] [CrossRef]
- Ding, J.C.; Mei, H.; Zheng, J.; Wang, Q.M.; Kang, M.C.; Zhang, T.F.; Kim, K.H. Microstructure and wettability of novel Al-containing diamond-like carbon films deposited by a hybrid sputtering system. J. Alloys Compd. 2021, 868, 159130. [Google Scholar] [CrossRef]
- Baba, K.; Hatada, R.; Flege, S.; Ensinger, W. Preparation and properties of Ag-containing diamond-like carbon films by magnetron plasma source ion implantation. Adv. Mater. Sci. Eng. 2012, 2012, 1–5. [Google Scholar] [CrossRef]
- Písařík, P.; Jelínek, M.; Remsa, J.; Mikšovský, J.; Zemek, J.; Jurek, K.; Šepitka, J. Antibacterial, mechanical and surface properties of Ag-DLC films prepared by dual PLD for medical applications. Mater. Sci. Eng. C 2017, 77, 955–962. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, J.; Li, H.; Ji, L.; Ye, Y.; Zhou, H. Preparation and properties of Ag/DLC nanocomposite films fabricated by unbalanced magnetron sputtering. Appl. Surf. Sci. 2013, 284, 165–170. [Google Scholar] [CrossRef]
- Jing, P.P.; Ma, D.L.; Gong, Y.L.; Luo, X.Y.; Zhang, Y.; Weng, Y.J.; Leng, Y.X. Influence of Ag doping on the microstructure, mechanical properties, and adhesion stability of diamond-like carbon films. Surf. Coat. Technol. 2021, 405, 126542. [Google Scholar] [CrossRef]
- Jing, P.P.; Gong, Y.L.; Deng, Q.Y.; Zhang, Y.Z.; Huang, N.; Leng, Y.X. The formation of the “rod-like wear debris” and tribological properties of Ag-doped diamond-like carbon films fabricated by a high-power pulsed plasma vapor deposition technique. Vacuum 2020, 173, 109125. [Google Scholar] [CrossRef]
- Constantinou, M.; Pervolaraki, M.; Nikolaou, P.; Prouskas, C.; Patsalas, P.; Kelires, P.; Constantinides, G. Microstructure and nanomechanical properties of pulsed excimer laser deposited DLC: Ag films: Enhanced nanotribological response. Surf. Coat. Technol. 2017, 309, 320–330. [Google Scholar] [CrossRef]
- Yu, X.; Qin, Y.; Wang, C.B.; Yang, Y.Q.; Ma, X.C. Effects of nanocrystalline silver incorporation on sliding tribological properties of Ag-containing diamond-like carbon films in multi-ion beam assisted deposition. Vacuum 2013, 89, 82–85. [Google Scholar] [CrossRef]
- Sahay, S.; Pandey, M.K.; Kar, A.K. Metal concentration dependent mechanical properties of electrodeposited nickel incorporated diamond like carbon (Ni-DLC) thin films studied by nanoindentation. Appl. Surf. Sci. 2019, 489, 73–79. [Google Scholar] [CrossRef]
- Pandey, B.; Pal, P.P.; Bera, S.; Ray, S.K.; Kar, A.K. Effect of nickel incorporation on microstructural and optical properties of electrodeposited diamond like carbon (DLC) thin films. Appl. Surf. Sci. 2012, 261, 789–799. [Google Scholar] [CrossRef]
- Khun, N.W.; Liu, E.; Yang, G.C. Structure, scratch resistance and corrosion performance of nickel doped diamond-like carbon thin films. Surf. Coat. Technol. 2010, 204, 3125–3130. [Google Scholar] [CrossRef]
- Wan, S.; Yu, Y.; Zhang, J.; Wang, L. Morphology and microstructure of patterned nickel incorporated amorphous carbon films by simple pyrolysis. Appl. Surf. Sci. 2010, 256, 4873–4878. [Google Scholar] [CrossRef]
- Ma, K.; Yang, G.; Yu, L.; Zhang, P. Synthesis and characterization of nickel-doped diamond-like carbon film electrodeposited at a low voltage. Surf. Coat. Technol. 2010, 204, 2546–2550. [Google Scholar] [CrossRef]
- Dai, W.; Kwon, S.H.; Wang, Q.; Liu, J. Influence of frequency and C2H2 flow on growth properties of diamond-like carbon coatings with AlCrSi co-doping deposited using a reactive high power impulse magnetron sputtering. Thin Solid Films 2018, 647, 26–32. [Google Scholar] [CrossRef]
- Sun, L.; Guo, P.; Ke, P.; Li, X.; Wang, A. Synergistic effect of Cu/Cr co-doping on the wettability and mechanical properties of diamond-like carbon films. Diam. Relat. Mater. 2016, 68, 1–9. [Google Scholar] [CrossRef]
- Dai, W.; Liu, J.; Geng, D.; Guo, P.; Zheng, J.; Wang, Q. Microstructure and property of diamond-like carbon films with Al and Cr co-doping deposited using a hybrid beams system. Appl. Surf. Sci. 2016, 388, 503–509. [Google Scholar] [CrossRef]
- Wei, X.; Chen, L.; Zhang, M.; Lu, Z.; Zhang, G. Effect of dopants (F, Si) material on the structure and properties of hydrogenated DLC film by plane cathode PECVD. Diam. Relat. Mater. 2020, 110, 108102. [Google Scholar] [CrossRef]
- Liu, X.; Yang, J.; Hao, J.; Zheng, J.; Gong, Q.; Liu, W. Microstructure, mechanical and tribological properties of Si and Al co-doped hydrogenated amorphous carbon films deposited at various bias voltages. Surf. Coat. Technol. 2012, 206, 4119–4125. [Google Scholar] [CrossRef]
- Pauleau, Y. Residual stresses in DLC films and adhesion to various substrates. Tribol. Diam.-Like Carbon Film. Fundam. Appl. 2008, 102–136. [Google Scholar] [CrossRef]
- Ritala, M.; Leskelä, M. Handbook of Thin Film Materials; Nalwa, H.S., Ed.; Academic Press: San Diego, CA, USA, 2001; Volume 1, Chapter 2. [Google Scholar]
- Caro, M.A.; Deringer, V.L.; Koskinen, J.; Laurila, T.; Csányi, G. Growth mechanism and origin of high s p 3 content in tetrahedral amorphous carbon. Phys. Rev. Lett. 2018, 120, 166101. [Google Scholar] [CrossRef] [PubMed]
- Vermeeren, P.; van Zeist, W.J.; Hamlin, T.A.; Fonseca Guerra, C.; Bickelhaupt, F.M. Not Carbon s–p Hybridization, but Coordination Number Determines C− H and C− C Bond Length. Chem. A Eur. J. 2021, 27, 7074–7079. [Google Scholar] [CrossRef] [PubMed]
- Pauling, L. The nature of the chemical bond. Application of results obtained from the quantum mechanics and from a theory of paramagnetic susceptibility to the structure of molecules. J. Am. Chem. Soc. 1931, 53, 1367–1400. [Google Scholar] [CrossRef]
- Pauling, L. General Chemistry; W.H. Freeman and Company: San Francisco, CA, USA, 1970. [Google Scholar]
- Hoffmann, R. Solids and Surfaces: A Chemist’s View of Bonding in Extended Structures; VCH Public Inc.: New York, NY, USA, 1988. [Google Scholar]
- Choi, J.H.; Lee, S.C.; Lee, K.R. A first-principles study on the bond characteristics in carbon containing Mo, Ag, or Al impurity atoms. Carbon 2008, 46, 185–188. [Google Scholar] [CrossRef]
- Wang, A.Y.; Ahn, H.S.; Lee, K.R.; Ahn, J.P. Unusual stress behavior in W-incorporated hydrogenated amorphous carbon films. Appl. Phys. Lett. 2005, 86, 111902. [Google Scholar] [CrossRef]
- Li, X.; Guo, P.; Sun, L.; Zuo, X.; Zhang, D.; Ke, P.; Wang, A. Ti/Al co-doping induced residual stress reduction and bond structure evolution of amorphous carbon films: An experimental and ab initio study. Carbon 2017, 111, 467–475. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Lee, K.R.; Wang, A. Effect of metal doping on structural characteristics of amorphous carbon system: A first-principles study. Thin Solid Film. 2016, 607, 67–72. [Google Scholar] [CrossRef]
- Ji, L.; Wu, Y.; Li, H.; Song, H.; Liu, X.; Ye, Y.; Liu, L. The role of trace Ti concentration on the evolution of microstructure and properties of duplex doped Ti (Ag)/DLC films. Vacuum 2015, 115, 23–30. [Google Scholar] [CrossRef]
- Huang, B.; Zhou, Q.; Zhang, E.G. Effect of thickness on tribological behavior of hydrogen free diamond-like carbon coating. Coatings 2020, 10, 243. [Google Scholar] [CrossRef]
- Song, R.; Chen, S.; Liu, Z.; Huo, C.; Chen, Q. Effect of W-doping on the structure and properties of DLC films prepared by combining physical and chemical vapor deposition. Diam. Relat. Mater. 2023, 132, 109687. [Google Scholar] [CrossRef]
- Schiøtz, J.; Di Tolla, F.D.; Jacobsen, K.W. Softening of nanocrystalline metals at very small grain sizes. Nature 1998, 391, 561–563. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, D.; Fu, Y.; Du, H. Toughening of hard nanostructural thin films: A critical review. Surf. Coat. Technol. 2005, 198, 2–8. [Google Scholar] [CrossRef]
- Streletskiy, O.A.; Zavidovskiy, I.A.; Balabanyan, V.Y.; Tsiskarashvili, A.V. Antibacterial properties of modified aC and ta-C coatings: The effects of the sp2/sp3 ratio, oxidation, nitridation, and silver incorporation. Appl. Phys. A 2022, 128, 929. [Google Scholar] [CrossRef]
- Marton, M.; Vojs, M.; Zdravecká, E.; Himmerlich, M.; Hänsel, T.; Krischok, S.; Redhammer, R. Raman spectroscopy of amorphous carbon prepared by pulsed arc discharge in various gas mixtures. J. Spectrosc. 2013, 2013, 467079. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Sun, W.C.; Dong, Y.R.; Ma, M.; Liu, Y.W.; Tian, S.S.; Jia, Y.P. Electrodeposition and microstructure of Ni and B co-doped diamond-like carbon (Ni/B-DLC) films. Surf. Coat. Technol. 2021, 405, 126713. [Google Scholar] [CrossRef]
- Qiang, L.; Gao, K.; Zhang, L.; Wang, J.; Zhang, B.; Zhang, J. Further improving the mechanical and tribological properties of low content Ti-doped DLC film by W incorporating. Appl. Surf. Sci. 2015, 353, 522–529. [Google Scholar] [CrossRef]
- Holleck, H.; Schier, A.V. Multilayer PVD coatings for wear protection. Surf. Coat. Technol. 1995, 76, 328–336. [Google Scholar] [CrossRef]
- Nieh, T.G.; Wadsworth, J. Hall-Petch relation in nanocrystalline solids. Scr. Metall. Et Mater. 1991, 25, 955–958. [Google Scholar] [CrossRef]
- Yang, W.; Guo, Y.; Xu, D.; Li, J.; Wang, P.; Ke, P.; Wang, A. Microstructure and properties of (Cr: N)-DLC films deposited by a hybrid beam technique. Surf. Coat. Technol. 2015, 261, 398–403. [Google Scholar] [CrossRef]
- Zhang, S.; Yue, W.; Kang, J.; Wang, Y.; Fu, Z.; Zhu, L.; Wang, C. Ti content on the tribological properties of W/Ti-doped diamond-like carbon film lubricating with additives. Wear 2019, 430, 137–144. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, P.; Sun, L.; Liu, L.; Xu, X.; Li, W.; Wang, A. Microstructure and property evolution of diamond-like carbon films co-doped by Al and Ti with different ratios. Surf. Coat. Technol. 2019, 361, 83–90. [Google Scholar] [CrossRef]
- Kong, C.; Guo, P.; Sun, L.; Zhou, Y.; Liang, Y.; Li, X.; Wang, A. Tribological mechanism of diamond-like carbon films induced by Ti/Al co-doping. Surf. Coat. Technol. 2018, 342, 167–177. [Google Scholar] [CrossRef]
- Sun, L.; Zuo, X.; Guo, P.; Li, X.; Ke, P.; Wang, A. Role of deposition temperature on the mechanical and tribological properties of Cu and Cr co-doped diamond-like carbon films. Thin Solid Films 2019, 678, 16–25. [Google Scholar] [CrossRef]
- Andersson, J.; Erck, R.A.; Erdemir, A. Friction of diamond-like carbon films in different atmospheres. Wear 2003, 254, 1070–1075. [Google Scholar] [CrossRef]
- Leyland, A.; Matthews, A. On the significance of the H/E ratio in wear control: A nanocomposite coating approach to optimised tribological behaviour. Wear 2000, 246, 1–11. [Google Scholar] [CrossRef]
- Gharam, A.; Lukitsch, M.J.; Balogh, M.P.; Irish, N.; Alpas, A.T. High temperature tribological behavior of W-DLC against aluminum. Surf. Coat. Technol. 2011, 206, 1905–1912. [Google Scholar] [CrossRef]
- Qi, Y.; Konca, E.; Alpas, A.T. Atmospheric effects on the adhesion and friction between non-hydrogenated diamond-like carbon (DLC) coating and aluminum–A first principles investigation. Surf. Sci. 2006, 600, 2955–2965. [Google Scholar] [CrossRef]
- Erdemir, A.; Donnet, C. Tribology of diamond-like carbon films: Recent progress and future prospects. J. Phys. D Appl. Phys. 2006, 39, R311. [Google Scholar] [CrossRef]
- Jing, P.P.; Feng, Q.G.; Lan, Q.H.; Ma, D.L.; Wang, H.Y.; Jiang, X.; Leng, Y.X. Migration and agglomeration behaviors of Ag nanocrystals in the Ag-doped diamond-like carbon film during its long-time service. Carbon 2023, 201, 648–658. [Google Scholar] [CrossRef]
- Richard, L.S.; Edward, J.S. Step motion on crystal surfaces. J. Appl. Phys. 1966, 37, 3682–3686. [Google Scholar]
- Ehrlich, G.; Hudda, F.G. Atomic view of surface self-diffusion: Tungsten on tungsten. J. Chem. Phys. 1966, 44, 1039–1049. [Google Scholar] [CrossRef]
- Sınmazçelik, T.; Yılmaz, T. Thermal aging effects on mechanical and tribological performance of PEEK and short fiber reinforced PEEK composites. Mater. Des. 2007, 28, 641–648. [Google Scholar] [CrossRef]
- Holmberg, K.; Ronkainen, H.; Laukkanen, A.; Wallin, K. Friction and wear of coated surfaces—Scales, modelling and simulation of tribomechanisms. Surf. Coat. Technol. 2007, 202, 1034–1049. [Google Scholar] [CrossRef]
- Wang, Y.; Su, Y.; Zhang, J.; Chen, Q.; Xu, J.; Bai, S.; Kubo, M. Reactive molecular dynamics simulations of wear and tribochemical reactions of diamond like carbon interfaces with nanoscale asperities under H2 gas: Implications for solid lubricant coatings. ACS Appl. Nano Mater. 2020, 3, 7297–7304. [Google Scholar] [CrossRef]
- Hatada, R.; Baba, K. Preparation of hydrophobic diamond like carbon films by plasma source ion implantation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1999, 148, 655–658. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Y.; Liu, L.; Guo, P.; Li, X.; Lee, K.R.; Wang, A. Corrosion behavior of diamond-like carbon film induced by Al/Ti co-doping. Appl. Surf. Sci. 2020, 509, 144877. [Google Scholar] [CrossRef]
- Ostrovskaya, L.; Perevertailo, V.; Ralchenko, V.; Dementjev, A.; Loginova, O. Wettability and surface energy of oxidized and hydrogen plasma-treated diamond films. Diam. Relat. Mater. 2002, 11, 845–850. [Google Scholar] [CrossRef]
- Rhee, S.K. Critical surface energies of Al2O3 and graphite. J. Am. Ceram. Soc. 1972, 55, 300–303. [Google Scholar] [CrossRef]
- Asl, A.M.; Kameli, P.; Ranjbar, M.; Salamati, H.; Jannesari, M. Correlations between microstructure and hydrophobicity properties of pulsed laser deposited diamond-like carbon films. Superlattices Microstruct. 2015, 81, 64–79. [Google Scholar]
- Miwa, M.; Nakajima, A.; Fujishima, A.; Hashimoto, K.; Watanabe, T. Effects of the surface roughness on sliding angles of water droplets on superhydrophobic surfaces. Langmuir 2000, 16, 5754–5760. [Google Scholar] [CrossRef]
- Meng, K.; Zhang, Z.; Tan, X.; Yu, Q. Microstructure evolution and wettability regulation of air-exposed hydrogen-free diamond-like carbon films. Diam. Relat. Mater. 2021, 120, 108609. [Google Scholar] [CrossRef]
- Samadi, M.; Eshaghi, A.; Bakhshi, S.R.; Aghaei, A.A. The influence of gas flow rate on the structural, mechanical, optical and wettability of diamond-like carbon thin films. Opt. Quantum Electron. 2018, 50, 1–14. [Google Scholar] [CrossRef]
- Wang, D.Y.; Chang, C.L.; Ho, W.Y. Oxidation behavior of diamond-like carbon films. Surf. Coat. Technol. 1999, 120, 138–144. [Google Scholar] [CrossRef]
- Yang, W.J.; Choa, Y.H.; Sekino, T.; Shim, K.B.; Niihara, K.; Auh, K.H. Thermal stability evaluation of diamond-like nanocomposite coatings. Thin Solid Film. 2003, 434, 49–54. [Google Scholar] [CrossRef]
- Absolom, D.R.; Lamberti, F.V.; Policova, Z.; Zingg, W.; van Oss, C.J.; Neumann, A.W. Surface thermodynamics of bacterial adhesion. Appl. Environ. Microbiol. 1983, 46, 90–97. [Google Scholar] [CrossRef]
- Busscher, H.J.; Weerkamp, A.H.; van der Mei, H.C.; Van Pelt, A.W.; de Jong, H.P.; Arends, J. Measurement of the surface free energy of bacterial cell surfaces and its relevance for adhesion. Appl. Environ. Microbiol. 1984, 48, 980–983. [Google Scholar] [CrossRef]
- Wang, J.; Huang, N.; Pan, C.J.; Kwok, S.C.H.; Yang, P.; Leng, Y.X.; Chu, P.K. Bacterial repellence from polyethylene terephthalate surface modified by acetylene plasma immersion ion implantation–deposition. Surf. Coat. Technol. 2004, 186, 299–304. [Google Scholar] [CrossRef]
- Marciano, F.R.; Bonetti, L.F.; Mangolin, J.F.; Da-Silva, N.S.; Corat, E.J.; Trava-Airoldi, V.J. Investigation into the antibacterial property and bacterial adhesion of diamond-like carbon films. Vacuum 2011, 85, 662–666. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, L.; Ogino, A.; Nagatsu, M. Investigation into the antibacterial property of carbon films. Diam. Relat. Mater. 2008, 17, 1416–1419. [Google Scholar] [CrossRef]
- Meister, T.L.; Fortmann, J.; Breisch, M.; Sengstock, C.; Steinmann, E.; Köller, M.; Ludwig, A. Nanoscale copper and silver thin film systems display differences in antiviral and antibacterial properties. Sci. Rep. 2022, 12, 7193. [Google Scholar] [CrossRef] [PubMed]
- Haji, S.H.; Ali, F.A.; Aka, S.T.H. Synergistic antibacterial activity of silver nanoparticles biosynthesized by carbapenem-resistant Gram-negative bacilli. Sci. Rep. 2022, 12, 1–13. [Google Scholar] [CrossRef]
- Rakowska, P.D.; Tiddia, M.; Faruqui, N.; Bankier, C.; Pei, Y.; Pollard, A.J.; Gilmore, I.S. Antiviral surfaces and coatings and their mechanisms of action. Commun. Mater. 2021, 2, 53. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Q.; Liu, Y.; Wang, S.; Abel, E.W. Reduction of bacterial adhesion on modified DLC coatings. Colloids Surf. B Biointerfaces 2008, 61, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Munawar, T.; Nadeem, M.S.; Mukhtar, F.; Azhar, A.; Hasan, M.; Mahmood, K.; Hussain, A.; Ali, A.; Arshad, M.; Nabi, M.A.U.; et al. Synthesis, characterization, and antibacterial study of novel Mg0.9Cr0.05M0.05O (M = Co, Ag, Ni) nanocrystals. Phys. B Condens. Matter 2021, 602, 412555. [Google Scholar] [CrossRef]
- Filova, E.; Vandrovcova, M.; Jelinek, M.; Zemek, J.; Houdkova, J.; Remsa, J.; Bacakova, L. Adhesion and differentiation of Saos-2 osteoblast-like cells on chromium-doped diamond-like carbon coatings. J. Mater. Sci. Mater. Med. 2017, 28, 1–14. [Google Scholar] [CrossRef]
- Hasebe, T.; Yohena, S.; Kamijo, A.; Okazaki, Y.; Hotta, A.; Takahashi, K.; Suzuki, T. Fluorine doping into diamond-like carbon coatings inhibits protein adsorption and platelet activation. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2007, 83, 1192–1199. [Google Scholar] [CrossRef]
- Tran, H.S.; Puc, M.M.; Hewitt, C.W.; Soll, D.B.; Marra, S.W.; Simonetti, V.A.; DelRossi, A.J. Diamond-like carbon coating and plasma or glow discharge treatment of mechanical heart valves. J. Investig. Surg. 1999, 12, 133–140. [Google Scholar]
- Gutensohn, K.; Beythien, C.; Bau, J.; Fenner, T.; Grewe, P.; Koester, R.; Padmanaban, K.; Kuehnl, P. In Vitro Analyses of Diamond-like Carbon Coated Stents: Reduction of Metal Ion Release, Platelet Activation, and Thrombogenicity. Thromb. Res. 2000, 99, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Airoldi, F.; Colombo, A.; Tavano, D.; Stankovic, G.; Klugmann, S.; Paolillo, V.; Di Mario, C. Comparison of diamond-like carbon-coated stents versus uncoated stainless steel stents in coronary artery disease. Am. J. Cardiol. 2004, 93, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Hauert, R. A review of modified DLC coatings for biological applications. Diam. Relat. Mater. 2003, 12, 583–589. [Google Scholar] [CrossRef]
- Sundfeldt, M.; Carlsson, L.; B Johansson, C.; Thomsen, P.; Gretzer, C. Aseptic loosening, not only a question of wear: A review of different theories. Acta Orthop. 2006, 77, 177–197. [Google Scholar] [CrossRef] [PubMed]
- Traisnel, M.; Le Maguer, D.; Hildebrand, H.F.; Iost, A. Corrosion of surgical implants. Clin. Mater. 1990, 5, 309–318. [Google Scholar] [CrossRef]
- McGeough, J.A. The Engineering of Human Joint Replacements; John Wiley Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Hee, A.C.; Zhao, Y.; Choudhury, D.; Ghosh, S.; Zhu, Q.; Zhu, H. Tribological behavior of hydrogenated diamond-like carbon on polished alumina substrate with chromium interlayer for biomedical application. Biotribology 2016, 7, 1–10. [Google Scholar] [CrossRef]
- Choudhury, D.; Urban, F.; Vrbka, M.; Hartl, M.; Krupka, I. A novel tribological study on DLC-coated micro-dimpled orthopedics implant interface. J. Mech. Behav. Biomed. Mater. 2015, 45, 121–131. [Google Scholar] [CrossRef]
- Choudhury, D.; Lackner, J.M.; Major, L.; Morita, T.; Sawae, Y.; Mamat, A.B.; Krupka, I. Improved wear resistance of functional diamond like carbon coated Ti–6Al–4V alloys in an edge loading conditions. J. Mech. Behav. Biomed. Mater. 2016, 59, 586–595. [Google Scholar] [CrossRef]
- Rothammer, B.; Schwendner, M.; Bartz, M.; Wartzack, S.; Böhm, T.; Krauß, S.; Marian, M. Wear Mechanism of Superhard Tetrahedral Amorphous Carbon (ta-C) Coatings for Biomedical Applications. Adv. Mater. Interfaces 2023, 10, 2202370. [Google Scholar] [CrossRef]
- Rothammer, B.; Neusser, K.; Marian, M.; Bartz, M.; Krauß, S.; Böhm, T.; Thiele, S.; Merle, B.; Detsch, R.; Wartzack, S. Amorphous Carbon Coatings for Total Knee Replacements—Part I: Deposition, Cytocompatibility, Chemical and Mechanical Properties. Polymers 2021, 13, 1952. [Google Scholar] [CrossRef]
- Rothammer, B.; Marian, M.; Neusser, K.; Bartz, M.; Böhm, T.; Krauß, S.; Wartzack, S. Amorphous carbon coatings for total knee replacements—Part II: Tribological behavior. Polymers 2021, 13, 1880. [Google Scholar] [CrossRef] [PubMed]
- Escudeiro, A.; Wimmer, M.A.; Polcar, T.; Cavaleiro, A. Tribological behavior of uncoated and DLC-coated CoCr and Ti-alloys in contact with UHMWPE and PEEK counterbodies. Tribol. Int. 2015, 89, 97–104. [Google Scholar] [CrossRef]
- Hauert, R.; Thorwarth, K.; Thorwarth, G. An overview on diamond-like carbon coatings in medical applications. Surf. Coat. Technol. 2013, 233, 119–130. [Google Scholar] [CrossRef]
- Jedrzejczak, A.; Szymanski, W.; Kolodziejczyk, L.; Sobczyk-Guzenda, A.; Kaczorowski, W.; Grabarczyk, J.; Batory, D. Tribological characteristics of aC: H: Si and aC: H: SiOx coatings tested in simulated body fluid and protein environment. Materials 2022, 15, 2082. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Tan, X.; Sui, X.; He, J.; Chen, C.; Hao, J.; Liu, W. Comparative study on microstructure, bio-tribological behavior and cytocompatibility of Cr-doped amorphous carbon films for Co–Cr–Mo artificial lumbar disc. Tribol. Int. 2021, 155, 106760. [Google Scholar] [CrossRef]
- Choudhury, D.; Lackner, J.; Fleming, R.A.; Goss, J.; Chen, J.; Zou, M. Diamond-like carbon coatings with zirconium-containing interlayers for orthopedic implants. J. Mech. Behav. Biomed. Mater. 2017, 68, 51–61. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, K.; Han, J.H.; Hwang, Y.H.; Xu, S.; Kim, D.E. One-step method to enhance biotribological properties and biocompatibility of DLC coating by ion beam irradiation. Friction 2022, 10, 1114–1126. [Google Scholar] [CrossRef]
- Takemoto, S.; Kusudo, Y.; Tsuru, K.; Hayakawa, S.; Osaka, A.; Takashima, S. Selective protein adsorption and blood compatibility of hydroxy-carbonate apatites. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2004, 69, 544–551. [Google Scholar] [CrossRef]
- Jing, P.P.; Su, Y.H.; Li, Y.X.; Liang, W.L.; Leng, Y.X. Mechanism of protein biofilm formation on Ag-DLC films prepared for application in joint implants. Surf. Coat. Technol. 2021, 422, 127553. [Google Scholar] [CrossRef]
- Dwivedi, N.; Kumar, S.; Singh, S.; Malik, H.K. Oxygen modified diamond-like carbon as window layer for amorphous silicon solar cells. Sol. Energy 2012, 86, 220–230. [Google Scholar] [CrossRef]
- Litovchenko, V.G.; Klyui, N.I. Solar cells based on DLC film–Si structures for space application. Sol. Energy Mater. Sol. Cells 2001, 68, 55–70. [Google Scholar] [CrossRef]
- Atwater, H.A.; Polman, A. Plasmonics for improved photovoltaic devices. Nat. Mater. 2010, 9, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.P.; Friedmann, T.A.; Dunn, R.G.; Stechel, E.B.; Schultz, P.A.; Siegal, M.P.; Missert, N. The electronic transport mechanism in amorphous tetrahedrally-coordinated carbon films. MRS Online Proc. Libr. 1997, 498, 97–102. [Google Scholar]
- Grill, A. Electrical and optical properties of diamond-like carbon. Thin Solid Film. 1999, 355, 189–193. [Google Scholar] [CrossRef]
- Milne, W.I. Electronic devices from diamond-like carbon. Semicond. Sci. Technol. 2003, 18, S81. [Google Scholar] [CrossRef]
- Robertson, J. Electron field emission from diamond and diamond-like carbon for field emission displays. Carbon 1999, 37, 759–763. [Google Scholar] [CrossRef]
- Fraga, M.A.; Furlan, H.; Pessoa, R.S.; Rasia, L.A.; Mateus, C.F.R. Studies on SiC, DLC and TiO2 thin films as piezoresistive sensor materials for high temperature application. Microsyst. Technol. 2012, 18, 1027–1033. [Google Scholar] [CrossRef]
- Zhu, H.; Xie, D.; Lin, S.; Zhang, W.; Yang, Y.; Zhang, R.; Tang, Y. Elastic loading enhanced NH3 sensing for surface acoustic wave sensor with highly porous nitrogen doped diamond like carbon film. Sens. Actuators B Chem. 2021, 344, 130175. [Google Scholar] [CrossRef]
- Iwata, T.; Oikawa, M.; Owashi, M.; Mihara, Y.; Ito, K.; Ninomiya, Y.; Kubota, S. The Verification of Engine Analysis Model Accuracy by Measuring Oil Film Pressure in the Main Bearings of a Motorcycle High-Speed Engine Using a Thin-Film Sensor. Lubricants 2022, 10, 314. [Google Scholar] [CrossRef]
- Aslan, N.; Kurt, M.Ş.; Koç, M.M. Silver-Doped Diamond-Like Carbon (DLC: Ag) Nanocomposite Films for Solar Tracking Applications. J. Electron. Mater. 2023, 52, 1–12. [Google Scholar] [CrossRef]
- Nauryzbekova, S.; Nussupov, K.; Bakranova, D. Simulation of Antireflective coatings system based on Porous Si/DLC and SiO2/TiO2 for Si solar cells. Mater. Today Proc. 2022, 49, 2474–2477. [Google Scholar] [CrossRef]
- Hekmat, M.; Shafiekhani, A.; Khabir, M. Near field and far field plasmonic enhancements with bilayers of different dimensions AgNPs@ DLC for improved current density in silicon solar. Sci. Rep. 2022, 12, 19663. [Google Scholar] [CrossRef] [PubMed]
- Tremmel, S.; Luo, X.; Rothammer, B.; Seynstahl, A.; Wang, B.; Rosenkranz, A.; Zhu, L. Evaluation of DLC, MoS2, and Ti3C2Tx thin films for triboelectric nanogenerators. Nano Energy 2022, 97, 107185. [Google Scholar] [CrossRef]
- Wang, Z.L.; Wang, A.C. On the origin of contact-electrification. Mater. Today 2019, 30, 34–51. [Google Scholar] [CrossRef]
- Wang, Z.L. On the first principle theory of nanogenerators from Maxwell’s equations. Nano Energy 2020, 68, 104272. [Google Scholar] [CrossRef]
- Mycielski, W.; Staryga, E.; Lipiński, A.; Mitura, S.; Sokołowska, A. Open-circuit mode drift mobility measurements in DLC films. Diam. Relat. Mater. 1994, 3, 858–860. [Google Scholar] [CrossRef]
- Ramaswamy, S.H.; Shimizu, J.; Chen, W.; Kondo, R.; Choi, J. Investigation of diamond-like carbon films as a promising dielectric material for triboelectric nanogenerator. Nano Energy 2019, 60, 875–885. [Google Scholar] [CrossRef]
- Miyagawa, S.; Nakao, S.; Choi, J.; Ikeyama, M.; Miyagawa, Y. Electrically conductive diamond-like carbon coatings prepared by plasma-based ion implantation with bipolar pulses. New Diam. Front. Carbon Technol. 2006, 16, 33–38. [Google Scholar]
- Ramaswamy, S.H.; Kondo, R.; Chen, W.; Fukushima, I.; Choi, J. Development of highly durable sliding triboelectric nanogenerator using diamond-like carbon films. Tribol. Online 2020, 15, 89–97. [Google Scholar] [CrossRef]
- Watson, P.K.; Yu, Z.Z. The contact electrification of polymers and the depth of charge penetration. J. Electrost. 1997, 40, 67–72. [Google Scholar] [CrossRef]
- Gao, M.; Kim, S.B.; Li, Y.; Ramaswamy, S.H.; Choi, J. Triboelectric nanogenerator with enhanced output and durability based on Si-DLC films. Nano Energy 2023, 105, 107997. [Google Scholar] [CrossRef]
- Choi, J.; Kawaguchi, M.; Kato, T.; Ikeyama, M. Deposition of Si-DLC film and its microstructural, tribological and corrosion properties. Microsyst. Technol. 2007, 13, 1353–1358. [Google Scholar] [CrossRef]
- Firdous, I.; Fahim, M.; Daoud, W.A. Performance enhancement of triboelectric nanogenerator through hole and electron blocking layers-based interfacial design. Nano Energy 2021, 82, 105694. [Google Scholar] [CrossRef]
- Lai, M.; Cheng, L.; Xi, Y.; Wu, Y.; Hu, C.; Guo, H.; Liu, R. Enhancing the performance of NaNbO3 triboelectric nanogenerators by dielectric modulation and electronegative modification. J. Phys. D: Appl. Phys. 2017, 51, 015303. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mitra, M.K.; Chattopadhyay, K.K. Low-macroscopic field emission from silicon-incorporated diamond-like carbon film synthesized by dc PECVD. Appl. Surf. Sci. 2007, 253, 5480–5484. [Google Scholar] [CrossRef]


















| Doping Element and Preparation Method | Doping Content (at.%) | Hardness (GPa) | Elastic Modulus (GPa) | Residual Stress (GPa) | ID/IG | Friction Coefficient | Wear Rate (mm3/N·m) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Al-1 Unbalance reactive magnetron sputtering | 0 | ~22.5 | ~1.37 | ~1.69 | ~0.07 | ~4.9 × 10−7 | [36] | |
| 0.37 | ~22.2 | ~1.34 | ~1.71 | ~0.068 | ~4.3 × 10−7 | |||
| 2.3 | ~19.8 | ~0.73 | ~1.78 | ~0.06 | 3.1 × 10−7 | |||
| 7.8 | ~9.9 | ~0.48 | ~1.94 | ~0.085 | ~2 × 10−6 | |||
| 19.3 | ~6.2 | ~0.34 | ~2.15 | ~0.12 | ~2.5 × 10−6 | |||
| Al-2 ALIS and magnetron sputtering | 0 | ~24.6 | ~228 | ~2.65 | ~0.43 | 0.113 | ~1.5 × 10−8 | [35] |
| 0.68 | ~17.5 | ~155 | ~1.6 | ~0.94 | 0.089 | ~6.4 × 10−8 | ||
| 6.93 | ~11.6 | ~127 | ~0.65 | ~1.23 | ~0.032 | ~7.6 × 10−8 | ||
| 11.04 | ~11 | ~125 | ~0.7 | ~1.28 | ~0.026 | ~1.05 × 10−7 | ||
| 17.6 | ~8.5 | ~118 | ~0.2 | ~1.41 | 0.024 | ~1.27 × 10−7 | ||
| Cu-1 High-power impulse magnetron sputtering | 0 | ~21.2 | ~140 | ~2.24 | 0.84 | ~0.125 | ~1.2 × 10−7 | [75] |
| 3.19 | ~19.5 | ~113 | ~1.8 | 0.93 | ~0.105 | ~4.6 × 10−8 | ||
| 8.21 | ~17.4 | ~111 | ~1.75 | 1.06 | ~0.1 | ~4.1 × 10−8 | ||
| 11.28 | ~15 | ~106 | ~1.3 | 1.10 | ~0.085 | ~1.9 × 10−8 | ||
| Ag-1 High-power pulsed plasma vapor deposition | 0 | ~8.6 | ~0.5 | ~0.12 | ~1.18 × 10−6 | [91] | ||
| 0.4 | ~8.8 | ~0.75 | ~0.17 | ~2.8 × 10−7 | ||||
| 2.41 | ~11 | ~0.83 | ~0.2 | ~1.1 × 10−7 | ||||
| 2.99 | ~12.8 | ~1.3 | ~0.15 | ~6 × 10−8 | ||||
| Ag-2 DC magnetron sputtering | 0 | 12.9 | 2.3 | 1.5 | 0.1 | ~0.075 × 10−6 | [37] | |
| 1.3 | 13.0 | 2.4 | 1.4 | 0.12 | ~0.1 × 10−6 | |||
| 6.1 | 12.0 | 2.2 | 1.6 | 0.13 | ~0.13 × 10−6 | |||
| 13.1 | 9.3 | 1.4 | 2.0 | 0.2 | ~0.19 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Yang, L.; Wu, H.; Zhao, L. Effects of Element Doping on the Structure and Properties of Diamond-like Carbon Films: A Review. Lubricants 2023, 11, 186. https://doi.org/10.3390/lubricants11040186
Sun H, Yang L, Wu H, Zhao L. Effects of Element Doping on the Structure and Properties of Diamond-like Carbon Films: A Review. Lubricants. 2023; 11(4):186. https://doi.org/10.3390/lubricants11040186
Chicago/Turabian StyleSun, Haibo, Lv Yang, Huaichao Wu, and Limei Zhao. 2023. "Effects of Element Doping on the Structure and Properties of Diamond-like Carbon Films: A Review" Lubricants 11, no. 4: 186. https://doi.org/10.3390/lubricants11040186
APA StyleSun, H., Yang, L., Wu, H., & Zhao, L. (2023). Effects of Element Doping on the Structure and Properties of Diamond-like Carbon Films: A Review. Lubricants, 11(4), 186. https://doi.org/10.3390/lubricants11040186