Mechano-Chemical Properties and Tribological Performance of Thin Perfluoropolyether (PFPE) Lubricant Film under Environmental Contaminants
Abstract
:1. Introduction
2. Methods
2.1. ReaxFF Forcefield
2.2. Material Preparations and Contaminant Adsorption
2.2.1. Lubricant Preparation
2.2.2. Substrate and Head Preparation
2.2.3. Contaminant Deposition
2.3. Airshear Simulation
2.4. Sliding Contact Simulation and Surface Contamination
3. Results and Discussion
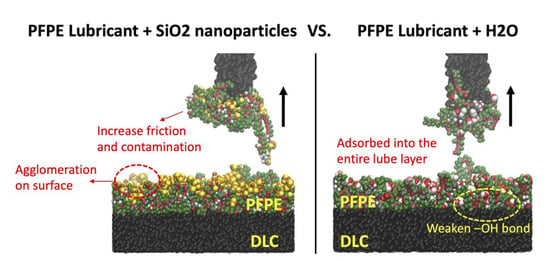
3.1. Adsorption of Silicon Dioxide (SiO2) and Water (H2O) Contaminants
3.2. Quantification of Airshear Behavior
3.3. Analysis of Radial Distribution Functions (RDFs) and the Potential Energy Surface (PES)
3.4. Quantification of Lubricant Degradation with Water (H2O) Contaminants
3.5. Friction Force Measurement during Sliding Contact
3.6. Surface Contamination by Lubricant Pick-Up
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Friedman, B.A.; Bain, J.A. Simulation of a Thermally Efficient HAMR Ridge Waveguide NFT on an AIN Heat Sink. In Proceedings of the 2021 IEEE 32nd Magnetic Recording Conference (TMRC), Virtual, 16–19 August 2021; pp. 1–2. [Google Scholar]
- Rai, R.; Bhargava, P.; Knigge, B.; Murthy, A.N. A method for monitoring head media spacing change in a hard disk drive using an embedded contact sensor. Microsyst. Technol. 2020, 26, 3459–3467. [Google Scholar] [CrossRef]
- Kiely, J.D.; Jones, P.M.; Yang, Y.; Brand, J.L.; Anaya-Dufresne, M.; Fletcher, P.C.; Zavaliche, F.; Toivola, Y.; Duda, J.C.; Johnson, M.T. Write-Induced Head Contamination in Heat-Assisted Magnetic Recording. IEEE Trans. Magn. 2017, 53, 1–7. [Google Scholar] [CrossRef]
- Seo, Y.W.; Ovcharenko, A.; Bilich, D.; Talke, F.E. Experimental Investigation of Hydrocarbon Contamination at the Head–Disk Interface. Tribol. Lett. 2017, 65, 54. [Google Scholar] [CrossRef]
- Kasai, P.H.; Eng, F.P. Silicon oxide formation in the disk environment. J. Inf. Stor. Proc. Syst. 2000, 2, 125–128. [Google Scholar]
- Peng, D.X.; Chen, C.H.; Kang, Y.; Chang, Y.P.; Chang, S.Y. Size effects of SiO2 nanoparticles as oil additives on tribology of lubricant. Ind. Lubr. Tribol. 2010, 62, 111–120. [Google Scholar] [CrossRef]
- Qi, H.; Guo, Y.; Zhang, L.; Li, G.; Zhang, G.; Wang, T.; Wang, Q. Covalently attached mesoporous silica–ionic liquid hybrid nanomaterial as water lubrication additives for polymer-metal tribopair. Tribol. Int. 2018, 119, 721–730. [Google Scholar] [CrossRef]
- Wang, N.; Wang, H.; Ren, J.; Gao, G.; Chen, S.; Zhao, G.; Yang, Y.; Wang, J. Novel additive of PTFE@SiO2 core-shell nanoparticles with superior water lubricating properties. Mater. Des. 2020, 195, 109069. [Google Scholar] [CrossRef]
- Wang, N.; Wang, H.; Ren, J.; Gao, G.; Zhao, G.; Yang, Y.; Wang, J. High-efficient and environmental-friendly PTFE@SiO2 core-shell additive with excellent AW/EP properties in PAO6. Tribol. Int. 2021, 158, 106930. [Google Scholar] [CrossRef]
- Liu, Y.; He, H.; Yang, M.; Zhang, R.; Yu, S.; Yang, T.; Wang, W.; Liang, F. Novel concept of nano-additive design: PTFE@silica Janus nanoparticles for water lubrication. Friction 2023. [Google Scholar] [CrossRef]
- Liu, N.; Bogy, D.B. Particle Contamination on a Thermal Flying-Height Control Slider. Tribol. Lett. 2009, 37, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.C.; Raman, V.; Karis, T.E.; Yao, Y.Z. Flyability failures due to siloxanes at the head-disk interface revisited. IEEE Trans. Magn. 2007, 43, 2223–2225. [Google Scholar] [CrossRef]
- Liu, Q.K.; Li, L.Q.; Zhang, G.Y.; Shuai, C.J. Study on Failure Process of Silicon-Doped Amorphous Carbon by ReaxFF Molecular Dynamics Simulation for HAMR. Ieee Trans. Magn. 2020, 56, 7502706. [Google Scholar] [CrossRef]
- Zhao, Z.; Bhushan, B. Effect of environmental humidity on the friction/stiction and durability of lubricated magnetic thin-film disks. Part I Mech. Eng. J.-J. Eng. 1997, 211, 295–301. [Google Scholar] [CrossRef]
- Zhao, Z.M.; Bhushan, B. Tribological performance of PFPE and X-1P lubricants at head-disk interface. Part I. Experimental results. Tribol. Lett. 1999, 6, 129–139. [Google Scholar] [CrossRef]
- Zhao, Z.M.; Bhushan, B.; Kajdas, C. Tribological performance of PFPE and X-1P lubricants at head-disk interface. Part II. Mechanisms. Tribol. Lett. 1999, 6, 141–148. [Google Scholar] [CrossRef]
- Tyndall, G.W.; Waltman, R.J.; Pacansky, J. Effect of adsorbed water on perfluoropolyether-lubricated magnetic recording disks. J. Appl. Phys. 2001, 90, 6287–6296. [Google Scholar] [CrossRef]
- Karis, T.E. Water adsorption on thin film media. J. Colloid. Interf. Sci. 2000, 225, 196–203. [Google Scholar] [CrossRef]
- Dai, Q.; Vurens, G.; Luna, M.; Salmeron, M. Lubricant distribution on hard disk surfaces: Effect of humidity and terminal group reactivity. Langmuir 1997, 13, 4401–4406. [Google Scholar] [CrossRef]
- Liu, H.W.; Bhushan, B. Nanotribological characterization of molecularly thick lubricant films for applications to MEMS/NEMS by AFM. Ultramicroscopy 2003, 97, 321–340. [Google Scholar] [CrossRef]
- Rahman, S.; Purani, D.; Ali, S.; Yeo, C.D. Effects of SiO2 Contaminant on Thermo-Mechanical/Chemical Properties and Lubricity of PFPE Lubricants. Lubricants 2021, 9, 90. [Google Scholar] [CrossRef]
- Li, L.; Jones, P.M.; Hsia, Y.T. Effect of chemical structure and molecular weight on high-temperature stability of some Fomblin Z-type lubricants. Tribol. Lett. 2004, 16, 21–27. [Google Scholar] [CrossRef]
- Bhushan, B. Magnetic Media Tribology—State-of-the-Art and Future Challenges. Wear 1990, 136, 169–197. [Google Scholar] [CrossRef]
- O’Hagan, D. Understanding organofluorine chemistry. An introduction to the C-F bond. Chem. Soc. Rev. 2008, 37, 308–319. [Google Scholar] [CrossRef]
- Erdemir, A.; Ramirez, G.; Eryilmaz, O.L.; Narayanan, B.; Liao, Y.F.; Kamath, G.; Sankaranarayanan, S.K.R.S. Carbon-based tribofilms from lubricating oils. Nature 2016, 536, 67–71. [Google Scholar] [CrossRef]
- Guo, X.C.; Knigge, B.; Marchon, B.; Waltman, R.J.; Carter, M.; Burns, J. Multidentate functionalized lubricant for ultralow head/disk spacing in a disk drive. J. Appl. Phys. 2006, 100, 044306. [Google Scholar] [CrossRef]
- Ghahri Sarabi, M.S.; Bogy, D.B. Simulation of the Performance of Various PFPE Lubricants Under Heat Assisted Magnetic Recording Conditions. Tribol. Lett. 2014, 56, 293–304. [Google Scholar] [CrossRef]
- Xiong, S.; Bogy, D.B. Experimental Study of Head-Disk Interface in Heat-Assisted Magnetic Recording. IEEE Trans. Magn. 2014, 50, 148–154. [Google Scholar] [CrossRef]
- Sakhalkar, S.V.; Bogy, D.B. A Model for Lubricant Transfer from Media to Head during Heat-Assisted Magnetic Recording (HAMR) Writing. Tribol. Lett. 2017, 65, 166. [Google Scholar] [CrossRef]
- Chen, X.; Kawai, K.; Zhang, H.; Fukuzawa, K.; Koga, N.; Itoh, S.; Azuma, N. ReaxFF Reactive Molecular Dynamics Simulations of Mechano-Chemical Decomposition of Perfluoropolyether Lubricants in Heat-Assisted Magnetic Recording. J. Phys. Chem. C 2020, 124, 22496–22505. [Google Scholar] [CrossRef]
- van Duin, A.C.T.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A reactive force field for hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar] [CrossRef] [Green Version]
- Chenoweth, K.; van Duin, A.C.T.; Goddard, W.A. ReaxFF reactive force field for molecular dynamics simulations of hydrocarbon oxidation. J. Phys. Chem. A 2008, 112, 1040–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashraf, C.; van Duin, A.C.T. Extension of the ReaxFF Combustion Force Field toward Syngas Combustion and Initial Oxidation Kinetics. J. Phys. Chem. A 2017, 121, 1051–1068. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Jaramillo-Botero, A.; Goddard, W.A.; Sun, H. Adaptive Accelerated ReaxFF Reactive Dynamics with Validation from Simulating Hydrogen Combustion. J. Am. Chem. Soc. 2014, 136, 13467. [Google Scholar] [CrossRef]
- Chipara, A.C.; Tsafack, T.; Owuor, P.S.; Yeon, J.; Junkermeier, C.E.; van Duin, A.C.T.; Bhowmick, S.; Asif, S.A.S.; Radhakrishnan, S.; Park, J.H.; et al. Underwater adhesive using solid-liquid polymer mixes. Mater. Today Chem. 2018, 9, 149–157. [Google Scholar] [CrossRef]
- Srinivasan, S.G.; van Duin, A.C.T. Molecular-Dynamics-Based Study of the Collisions of Hyperthermal Atomic Oxygen with Graphene Using the ReaxFF Reactive Force Field. J. Phys. Chem. A 2011, 115, 13269–13280. [Google Scholar] [CrossRef]
- Chenoweth, K.; Cheung, S.; van Duin, A.C.T.; Goddard, W.A.; Kober, E.M. Simulations on the thermal decomposition of a poly(dimethylsiloxane) polymer using the ReaxFF reactive force field. J. Am. Chem. Soc. 2005, 127, 7192–7202. [Google Scholar] [CrossRef] [Green Version]
- Thompson, A.P.; Aktulga, H.M.; Berger, R.; Bolintineanu, D.S.; Brown, W.M.; Crozier, P.S.; Veld, P.J.I.; Kohlmeyer, A.; Moore, S.G.; Nguyen, T.D.; et al. LAMMPS-a flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 2022, 271, 108171. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform 2012, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Chung, P.; Vemuri, S.; Jhon, M.S. Atomistic and Molecular Effects on the Surface Morphology and Film Conformation of Perfluoropolyether Lubricant Layer. IEEE Trans. Magn. 2015, 51, 3300904. [Google Scholar] [CrossRef]
- Song, J.A.; Talukder, S.; Rahman, S.M.; Jung, Y.N.; Yeo, C.D. Comparison study on surface and thermo-chemical properties of PFPE lubricants on DLC film through MD simulations. Tribol. Int. 2021, 156, 106835. [Google Scholar] [CrossRef]
- Apra, E.; Bylaska, E.J.; de Jong, W.A.; Govind, N.; Kowalski, K.; Straatsma, T.P.; Valiev, M.; van Dam, H.J.J.; Alexeev, Y.; Anchell, J.; et al. NWChem: Past, present, and future. J. Chem. Phys. 2020, 152, 184102. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A Gen. Phys. 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic Population Analysis on Lcao-Mo Molecular Wave Functions.1. J. Chem. Phys. 1955, 23, 1833–1840. [Google Scholar] [CrossRef] [Green Version]
- Hehre, W.J.; Stewart, R.F.; Pople, J.A. Self-Consistent Molecular-Orbital Methods.I. Use of Gaussian Expansions of Slater-Type Atomic Orbitals. J. Chem. Phys. 1969, 51, 2657–2664. [Google Scholar] [CrossRef]
- Collins, J.B.; Schleyer, P.V.; Binkley, J.S.; Pople, J.A. Self-Consistent Molecular-Orbital Methods.17. Geometries and Binding-Energies of 2nd-Row Molecules—Comparison of 3 Basis Sets. J. Chem. Phys. 1976, 64, 5142–5151. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. Model. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Galli, G.; Martin, R.M.; Car, R.; Parrinello, M. Structural and Electronic-Properties of Amorphous-Carbon. Phys. Rev. Lett. 1989, 62, 555–558. [Google Scholar] [CrossRef]
- Janke, W.; Schierz, P.; Zierenberg, J. Transition barrier at a first-order phase transition in the canonical and microcanonical ensemble. J. Phys. Conf. Ser. 2017, 921, 012018. [Google Scholar] [CrossRef] [Green Version]
- Bauer, B.A.; Patel, S. Properties of water along the liquid-vapor coexistence curve via molecular dynamics simulations using the polarizable TIP4P-QDP-LJ water model. J. Chem. Phys. 2009, 131, 084709. [Google Scholar] [CrossRef] [Green Version]
- Branco, R.J.; Graber, M.; Denis, V.; Pleiss, J. Molecular mechanism of the hydration of Candida antarctica lipase B in the gas phase: Water adsorption isotherms and molecular dynamics simulations. Chembiochem 2009, 10, 2913–2919. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Qiao, L. Molecular dynamics simulations of the surface tension of oxygen-supersaturated water. AIP Adv. 2017, 7, 045001. [Google Scholar] [CrossRef] [Green Version]
- Wu, L. Modeling and simulation of the interaction between lubricant droplets on the slider surface and air flow within the head/disk interface of disk drives. IEEE Trans. Magn. 2006, 42, 2480–2482. [Google Scholar] [CrossRef] [Green Version]
- SCM. AMS 2022.101. Available online: http://www.scm.com (accessed on 18 April 2022).
- Chill, S.T.; Welborn, M.; Terrell, R.; Zhang, L.; Berthet, J.C.; Pedersen, A.; Jonsson, H.; Henkelman, G. EON: Software for long time simulations of atomic scale systems. Model. Simul. Mater. Sc. 2014, 22, 055002. [Google Scholar] [CrossRef]
- Hunter:, J.D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]














| Lubricants + Contaminants | Percentage of Atoms Adsorbed (%) |
|---|---|
| Ztetraol + SiO2 at 300 K | 67.1% |
| Ztetraol + SiO2 at 700 K | 64.9% |
| ZTMD + SiO2 at 300 K | 66.3% |
| ZTMD + SiO2 at 700 K | 64.4% |
| Ztetraol + H2O at 300 K | 66.2% |
| Ztetraol + H2O at 700 K | 63.9% |
| ZTMD + H2O at 300 K | 58.2% |
| ZTMD + H2O at 700 K | 53.9% |
| ||||||
| Cbb–Cbb | Cbb–Fbb | Cbb–Hbb | Cbb–Obb | Cbb–Oend | Oend–Hend | |
| No. of Degraded bonds | 5 | 136 | 64 | 12 | 73 | 98 |
| % of Degradation | 3.06% | 0.52% | 7.62% | 0.36% | 30.42% | 40.83% |
| ||||||
| Cbb–Cbb | Cbb–Fbb | Cbb–Hbb | Cbb–Obb | Cbb–Oend | Oend–Hend | |
| No. of Degraded bonds | 5 | 136 | 64 | 12 | 73 | 98 |
| % of Degradation | 3.06% | 0.52% | 7.62% | 0.36% | 30.42% | 40.83% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, Y.; Yeo, C. Mechano-Chemical Properties and Tribological Performance of Thin Perfluoropolyether (PFPE) Lubricant Film under Environmental Contaminants. Lubricants 2023, 11, 306. https://doi.org/10.3390/lubricants11070306
Jung Y, Yeo C. Mechano-Chemical Properties and Tribological Performance of Thin Perfluoropolyether (PFPE) Lubricant Film under Environmental Contaminants. Lubricants. 2023; 11(7):306. https://doi.org/10.3390/lubricants11070306
Chicago/Turabian StyleJung, Yeonjin, and Changdong Yeo. 2023. "Mechano-Chemical Properties and Tribological Performance of Thin Perfluoropolyether (PFPE) Lubricant Film under Environmental Contaminants" Lubricants 11, no. 7: 306. https://doi.org/10.3390/lubricants11070306
APA StyleJung, Y., & Yeo, C. (2023). Mechano-Chemical Properties and Tribological Performance of Thin Perfluoropolyether (PFPE) Lubricant Film under Environmental Contaminants. Lubricants, 11(7), 306. https://doi.org/10.3390/lubricants11070306