Are Ionic Liquids Good Boundary Lubricants? A Molecular Perspective
Abstract
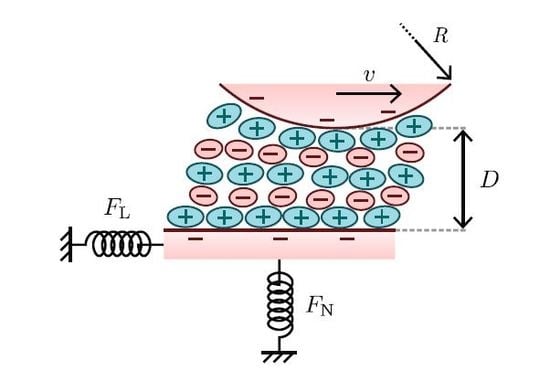
:1. Introduction
2. The Molecular Mechanisms of Friction across Ionic Liquids
3. Small Fractions of Molecular Liquid Can Dramatically Influence Lubrication of Ionic Liquids
4. Ionic Liquids Can Be Manipulated to Externally Switch Friction
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bowden, F.P.; Tabor, D. The Friction and Lubrication of Solids; Oxford University Press: Oxford, UK, 1950. [Google Scholar]
- Kardar, M.; Golestanian, R. The “friction” of vacuum, and other fluctuation-induced forces. Rev. Mod. Phys. 1999, 71, 1233–1245. [Google Scholar] [CrossRef]
- Bermúdez, M.D.; Jiménez, A.E.; Sanes, J.; Carrión, F.J. Ionic Liquids as Advanced Lubricant Fluids. Molecules 2009, 14, 2888–2908. [Google Scholar] [CrossRef] [PubMed]
- Minami, I. Ionic Liquids in Tribology. Molecules 2009, 14, 2286–2305. [Google Scholar] [CrossRef] [PubMed]
- Palacio, M.; Bhushan, B. A Review of Ionic Liquids for Green Molecular Lubrication in Nanotechnology. Tribol. Lett. 2010, 40, 247–268. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, M.V.; Kornyshev, A.A. Ionic Liquids at Electrified Interfaces. Chem. Rev. 2014, 114, 2978–3036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and Nanostructure in Ionic Liquids. Chem. Rev. 2015, 115, 6357–6426. [Google Scholar] [CrossRef] [PubMed]
- Perkin, S.; Albrecht, T.; Klein, J. Layering and shear properties of an ionic liquid, 1-ethyl-3-methylimidazolium ethylsulfate, confined to nano-films between mica surfaces. Phys. Chem. Chem. Phys. 2010, 12, 1243–1247. [Google Scholar] [CrossRef] [PubMed]
- Hjalmarsson, N.; Atkin, R.; Rutland, M.W. Is the boundary layer of an ionic liquid equally lubricating at higher temperature? Phys. Chem. Chem. Phys. 2016, 18, 9232–9239. [Google Scholar] [CrossRef] [PubMed]
- Israelachvili, J.N. Intermolecular and Surface Forces; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Horn, R.G.; Israelachvili, J.N. Direct measurement of structural forces between two surfaces in a nonpolar liquid. J. Chem. Phys. 1981, 75, 1400–1411. [Google Scholar] [CrossRef]
- Christenson, H.K. Experimental measurements of solvation forces in nonpolar liquids. J. Chem. Phys. 1983, 78, 6906–6913. [Google Scholar] [CrossRef]
- Israelachvili, J.N.; Pashley, R.M. Molecular layering of water at surfaces and origin of repulsive hydration forces. Nature 1983, 306, 249–250. [Google Scholar] [CrossRef]
- Horn, R.G.; Israelachvili, J.N. Molecular organization and viscosity of a thin film of molten polymer between two surfaces as probed by force measurements. Macromolecules 1988, 21, 2836–2841. [Google Scholar] [CrossRef]
- Horn, R.G.; Evans, D.F.; Ninham, B.W. Double-layer and solvation forces measured in a molten salt and its mixtures with water. J. Phys. Chem. 1988, 92, 3531–3537. [Google Scholar] [CrossRef]
- Atkin, R.; Warr, G.G. Structure in Confined Room-Temperature Ionic Liquids. J. Phys. Chem. C 2007, 111, 5162–5168. [Google Scholar] [CrossRef]
- Bou-Malham, I.; Bureau, L. Nanoconfined ionic liquids: Effect of surface charges on flow and molecular layering. Soft Matter 2010, 6, 4062–4065. [Google Scholar] [CrossRef]
- Ueno, K.; Kasuya, M.; Watanabe, M.; Mizukami, M.; Kurihara, K. Resonance shear measurement of nanoconfined ionic liquids. Phys. Chem. Chem. Phys. 2010, 12, 4066–4071. [Google Scholar] [CrossRef] [PubMed]
- Hayes, R.; Borisenko, N.; Tam, M.K.; Howlett, P.C.; Endres, F.; Atkin, R. Double Layer Structure of Ionic Liquids at the Au(111) Electrode Interface: An Atomic Force Microscopy Investigation. J. Phys. Chem. C 2011, 115, 6855–6863. [Google Scholar] [CrossRef]
- Perkin, S.; Crowhurst, L.; Niedermeyer, H.; Welton, T.; Smith, A.M.; Gosvami, N.N. Self-assembly in the electrical double layer of ionic liquids. Chem. Commun. 2011, 47, 6572–6574. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Lovelock, K.R.J.; Gosvami, N.N.; Licence, P.; Dolan, A.; Welton, T.; Perkin, S. Monolayer to Bilayer Structural Transition in Confined Pyrrolidinium-Based Ionic Liquids. J. Phys. Chem. Lett. 2013, 4, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Lovelock, K.R.J.; Gosvami, N.N.; Welton, T.; Perkin, S. Quantized friction across ionic liquid thin films. Phys. Chem. Chem. Phys. 2013, 15, 15317–15320. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Parkes, M.A.; Perkin, S. Molecular Friction Mechanisms Across Nanofilms of a Bilayer-Forming Ionic Liquid. J. Phys. Chem. Lett. 2014, 5, 4032–4037. [Google Scholar] [CrossRef] [PubMed]
- Israelachvili, J.N.; Kott, S.J. Shear Properties and Structure of Simple Liquids in Molecularly Thin Films: The Transition from Bulk (Continuum) to Molecular Behavior with Decreasing Film Thickness. J. Colloid Interface Sci. 1989, 129, 461–467. [Google Scholar] [CrossRef]
- Thompson, P.A.; Robbins, M.O. Origin of Stick-Slip Motion in Boundary Lubrication. Science 1990, 250, 792–794. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.; Kumacheva, E. Confinement-Induced Phase Transitions in Simple Liquids. Science 1995, 269, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.; Kumacheva, E. Simple liquids confined to molecularly thin layers. I. Confinement-induced liquid-to-solid phase transitions. J. Chem. Phys. 1998, 108, 6996–7009. [Google Scholar] [CrossRef]
- Alba-Simionesco, C.; Coasne, B.; Dosseh, G.; Dudziak, G.; Gubbins, K.E.; Radhakrishnan, R.; Sliwinska-Bartkowiak, M. Effects of confinement on freezing and melting. J. Phys. Condens. Matter 2006, 18, R15. [Google Scholar] [CrossRef] [PubMed]
- Klein, J. Frictional Dissipation in Stick-Slip Sliding. Phys. Rev. Lett. 2007, 98. [Google Scholar] [CrossRef] [PubMed]
- Docherty, H.; Cummings, P.T. Direct evidence for fluid-solid transition of nanoconfined fluids. Soft Matter 2010, 6, 1640–1643. [Google Scholar] [CrossRef]
- Lei, Y.; Leng, Y. Stick-Slip Friction and Energy Dissipation in Boundary Lubrication. Phys. Rev. Lett. 2011, 107, 147801. [Google Scholar] [CrossRef] [PubMed]
- Rosenhek-Goldian, I.; Kampf, N.; Yeredor, A.; Klein, J. On the question of whether lubricants fluidize in stick-slip friction. Proc. Natl. Acad. Sci. USA 2015, 112, 7117–7122. [Google Scholar] [CrossRef] [PubMed]
- Jee, A.; Lou, K.; Granick, S. Scrutinizing evidence of no dilatancy upon stick-slip of confined fluids. Proc. Natl. Acad. Sci. USA 2015, 112, 11139–11140. [Google Scholar] [CrossRef] [PubMed]
- Israelachvili, J.N.; Drummond, C. On the conformational state of molecules in molecularly thin shearing films. Proc. Natl. Acad. Sci. USA 2015, 112, E4973. [Google Scholar] [CrossRef] [PubMed]
- Rosenhek-Goldian, I.; Kampf, N.; Yeredor, A.; Klein, J. Reply to Jee et al. and Israelachvili and Drummond: Lubricant films do not fluidize in intermittent stick-slip friction. Proc. Natl. Acad. Sci. USA 2015, 112, E4974. [Google Scholar] [CrossRef] [PubMed]
- Werzer, O.; Cranston, E.D.; Warr, G.G.; Atkin, R.; Rutland, M.W. Ionic liquid nanotribology: Mica-silica interactions in ethylammonium nitrate. Phys. Chem. Chem. Phys. 2012, 14, 5147–5152. [Google Scholar] [CrossRef] [PubMed]
- Elbourne, A.; Sweeney, J.; Webber, G.B.; Wanless, E.J.; Warr, G.G.; Rutland, M.W.; Atkin, R. Adsorbed and near-surface structure of ionic liquids determines nanoscale friction. Chem. Commun. 2013, 49, 6797–6799. [Google Scholar] [CrossRef] [PubMed]
- Gee, M.L.; McGuiggan, P.M.; Israelachvili, J.N.; Homola, A.M. Liquid to solidlike transitions of molecularly thin films under shear. J. Chem. Phys. 1990, 93, 1895–1906. [Google Scholar] [CrossRef]
- Kumacheva, E.; Klein, J. Simple liquids confined to molecularly thin layers. II. Shear and frictional behavior of solidified films. J. Chem. Phys. 1998, 108, 7010–7022. [Google Scholar] [CrossRef]
- Espinosa-Marzal, R.M.; Arcifa, A.; Rossi, A.; Spencer, N.D. Microslips to “Avalanches” in Confined, Molecular Layers of Ionic Liquids. J. Phys. Chem. Lett. 2014, 5, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Lee, A.A.; Perkin, S. Switching the Structural Force in Ionic Liquid-Solvent Mixtures by Varying Composition. Phys. Rev. Lett. 2017, 118. [Google Scholar] [CrossRef] [PubMed]
- Hayes, R.; Warr, G.G.; Atkin, R. At the interface: Solvation and designing ionic liquids. Phys. Chem. Chem. Phys. 2010, 12, 1709–1723. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, J.; Webber, G.B.; Rutland, M.W.; Atkin, R. Effect of ion structure on nanoscale friction in protic ionic liquids. Phys. Chem. Chem. Phys. 2014, 16, 16651–16658. [Google Scholar] [CrossRef] [PubMed]
- Federici Canova, F.; Matsubara, H.; Mizukami, M.; Kurihara, K.; Shluger, A.L. Shear dynamics of nanoconfined ionic liquids. Phys. Chem. Chem. Phys. 2014, 16, 8247–8256. [Google Scholar] [CrossRef] [PubMed]
- Hoth, J.; Hausen, F.; Müser, M.H.; Bennewitz, R. Force microscopy of layering and friction in an ionic liquid. J. Phys. Condens. Matter 2014, 26. [Google Scholar] [CrossRef] [PubMed]
- De Wijn, A.S.; Fasolino, A.; Filippov, A.E.; Urbakh, M. Effects of molecule anchoring and dispersion on nanoscopic friction under electrochemical control. J. Phys. Condens. Matter 2016, 28. [Google Scholar] [CrossRef] [PubMed]
- Kramer, G.; Hausen, F.; Bennewitz, R. Dynamic shear force microscopy of confined liquids at a gold electrode. Faraday Discuss. 2017, 199, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, O.Y.; Bresme, F.; Kornyshev, A.A.; Urbakh, M. Water in Ionic Liquid Lubricants: Friend and Foe. ACS Nano 2017, 11, 6825–6831. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Marzal, R.M.; Arcifa, A.; Rossi, A.; Spencer, N.D. Ionic Liquids Confined in Hydrophilic Nanocontacts: Structure and Lubricity in the Presence of Water. J. Phys. Chem. C 2014, 118, 6491–6503. [Google Scholar] [CrossRef]
- Cheng, H.W.; Stock, P.; Moeremans, B.; Baimpos, T.; Banquy, X.; Renner, F.U.; Valtiner, M. Characterizing the Influence of Water on Charging and Layering at Electrified Ionic-Liquid/Solid Interfaces. Adv. Mater. Interfaces 2015, 2. [Google Scholar] [CrossRef]
- Thomaz, J.E.; Lawler, C.M.; Fayer, M.D. The Influence of Water on the Alkyl Region Structure in Variable Chain Length Imidazolium-Based Ionic Liquid/Water Mixtures. J. Phys. Chem. B 2016, 120, 10350–10357. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.U.; Glavatskih, S.; Antzutkin, O.N. Boron in Tribology: From Borates to Ionic Liquids. Tribol. Lett. 2013, 51, 281–301. [Google Scholar] [CrossRef]
- Dong, R.; Wen, P.; Zhang, S.; Zhang, C.; Sun, W.; Fan, M.; Yang, D.; Zhou, F.; Liu, W. The synthesis and tribological properties of dicarboxylic acid ionic liquids. Tribol. Int. 2017, 114, 132–140. [Google Scholar] [CrossRef]
- Sweeney, J.; Webber, G.B.; Atkin, R. Near surface properties of mixtures of propylammonium nitrate with n-alkanols 2. Nanotribology and fluid dynamics. Phys. Chem. Chem. Phys. 2015, 17, 26629–26637. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Somers, A.E.; Howlett, P.C.; Rutland, M.W.; Forsyth, M.; Atkin, R. Addition of low concentrations of an ionic liquid to a base oil reduces friction over multiple length scales: A combined nano- and macrotribology investigation. Phys. Chem. Chem. Phys. 2016, 18, 6541–6547. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Perkin, S. Influence of Lithium Solutes on Double-Layer Structure of Ionic Liquids. J. Phys. Chem. Lett. 2015, 6, 4857–4861. [Google Scholar] [CrossRef] [PubMed]
- Hjalmarsson, N.; Atkin, R.; Rutland, M.W. Effect of Lithium Ions on Rheology and Interfacial Forces in Ethylammonium Nitrate and Ethanolammonium Nitrate. J. Phys. Chem. C 2016, 120, 26960–26967. [Google Scholar] [CrossRef]
- Fajardo, O.Y.; Bresme, F.; Kornyshev, A.A.; Urbakh, M. Electrotunable Lubricity with Ionic Liquid Nanoscale Films. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, O.Y.; Bresme, F.; Kornyshev, A.A.; Urbakh, M. Electrotunable Friction with Ionic Liquid Lubricants: How Important Is the Molecular Structure of the Ions? J. Phys. Chem. Lett. 2015, 6, 3998–4004. [Google Scholar] [CrossRef] [PubMed]
- Capozza, R.; Benassi, A.; Vanossi, A.; Tosatti, E. Electrical charging effects on the sliding friction of a model nano-confined ionic liquid. J. Chem. Phys. 2015, 143, 144703. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, J.; Hausen, F.; Hayes, R.; Webber, G.B.; Endres, F.; Rutland, M.W.; Bennewitz, R.; Atkin, R. Control of Nanoscale Friction on Gold in an Ionic Liquid by a Potential-Dependent Ionic Lubricant Layer. Phys. Rev. Lett. 2012, 109, 155502. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Rutland, M.W.; Atkin, R. Ionic liquid lubrication: influence of ion structure, surface potential and sliding velocity. Phys. Chem. Chem. Phys. 2013, 15, 14616–14623. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Atkin, R.; Page, A.J. Combined friction force microscopy and quantum chemical investigation of the tribotronic response at the propylammonium nitrate-graphite interface. Phys. Chem. Chem. Phys. 2015, 17, 16047–16052. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wood, R.J.; Rutland, M.W.; Atkin, R. An ionic liquid lubricant enables superlubricity to be “switched on” in situ using an electrical potential. Chem. Commun. 2014, 50, 4368–4370. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Rutland, M.W.; Watanabe, M.; Atkin, R. Boundary layer friction of solvate ionic liquids as a function of potential. Faraday Discuss. 2017, 199, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Drummond, C. Electric-Flield-Ilnduced Flriction Rleduction and Clontrol. Phys. Rev. Lett. 2012, 109. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Nakano, K.; Kato, T.; Morishita, S. Control of friction coefficient by applying electric fields across liquid crystal boundary films. Wear 1994, 175, 143–149. [Google Scholar] [CrossRef]
- Nakano, S.; Mizukami, M.; Kurihara, K. Effect of confinement on electric field induced orientation of a nematic liquid crystal. Soft Matter 2014, 10, 2110–2115. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.K.; Li, H.; Rutland, M.W.; Webber, G.B.; Atkin, R. Tribotronic control of friction in oil-based lubricants with ionic liquid additives. Phys. Chem. Chem. Phys. 2016, 18, 23657–23662. [Google Scholar] [CrossRef] [PubMed]
- Bordes, R.; Marty, J.D.; Lauth-de Viguerie, N. Room-Temperature Zwitterionic Ionic Liquids. Fr. Ukr. J. Chem. 2016, 4, 85–94. [Google Scholar] [CrossRef]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lhermerout, R.; Diederichs, C.; Perkin, S. Are Ionic Liquids Good Boundary Lubricants? A Molecular Perspective. Lubricants 2018, 6, 9. https://doi.org/10.3390/lubricants6010009
Lhermerout R, Diederichs C, Perkin S. Are Ionic Liquids Good Boundary Lubricants? A Molecular Perspective. Lubricants. 2018; 6(1):9. https://doi.org/10.3390/lubricants6010009
Chicago/Turabian StyleLhermerout, Romain, Christophe Diederichs, and Susan Perkin. 2018. "Are Ionic Liquids Good Boundary Lubricants? A Molecular Perspective" Lubricants 6, no. 1: 9. https://doi.org/10.3390/lubricants6010009
APA StyleLhermerout, R., Diederichs, C., & Perkin, S. (2018). Are Ionic Liquids Good Boundary Lubricants? A Molecular Perspective. Lubricants, 6(1), 9. https://doi.org/10.3390/lubricants6010009