1. Introduction
Applications in which oil-water interfaces are inevitable, such as hydroelectric power plants, logging and mining industries, or sailing vessels, represent a potential hazard for the environment. Apart from the potential risk of an environmental catastrophe in the case of an accident, these applications cause regular spillage of lubricant under normal operation. Though a large amount of lubricants are recycled or burned, it is estimated that about 50% of the lubricants end up in the environment [
1]. The environmental consequences escalate when the lubricant is mixed with water since a small quantity of oil can contaminate a large amount of water.
In marine applications, lubricants are required in gear boxes, thrusters, controllable pitch propellers, stabilizers, stern tube bearings and seals, rudder bearings, dredges, and grabs. These mechanical components all have an oil-to-sea interface, representing a potential source of oil spillage to the aquatic environment. Even during normal sailing, there is a continuous lubricant spillage into the sea through the stern tube seals (
Figure 1) [
2]. This type of rotary lip seals operates under pressurized conditions and is designed to minimize both the lubricant leakage as well as the water ingress to the stern tube.
Governmental regulations limit the use of certain lubricants in applications where there is a risk of environmental damage. The German Blue Angel, the European Eco-label, and the American Vessel General Permit (VGP) labelling programs are the most well-known. In December 2013, the use of Environmentally Acceptable Lubricants (EALs) became mandatory in large ships sailing within the coastal waters of the United States [
3,
4]. To minimize seawater pollution, all oil-to-sea interfaces of sailing vessels must use these more environmentally friendly lubricants. Any lubricant can be labelled as an EAL as long as it meets certain criteria: biodegradability, minimum toxicity, and bioaccumulation potential. Consequently, a wide range of lubricants tailored to fit this new legislation have been introduced to the marine lubricants segment.
The American VGP 2013 only allows the following four kinds of base oils for the formulation of EALs: hydraulic oil environmental triglyceride (HETG), hydraulic oil environmental ester synthetic (HEES), hydraulic oil environmental polyglycol (HEPG or PAG), and poly-α-olefins including related hydrocarbon products (HEPR or PAO) [
4]. The first group are lubricants obtained from plants and animal fats. Although more economic than synthetic esters, their quick aging when exposed to water and heat makes them unsuitable for hydraulic systems [
4]. The stricter European Eco-Label program restricts the content of a high fraction of natural esters or synthetic esters made from renewable resources in the formulation of a marine lubricant [
5].
The second kind of base oils for EALs results from reacting carboxylic acids and alcohols to obtain synthetic esters (i.e., esterification reaction). These have been the obvious choice for most ship owners. These base oils can be specially tailored to the application by selecting the proper acids and alcohols to form specific esters. Synthetic esters are susceptible to hydrolysis and have compatibility problems with the elastomeric stern tube seals [
4].
Petroleum-based PAG’s are obtained from ethylene or propylene oxide via polymerisation. The proportion of these dictates their solubility in water (i.e., polarity). Their maximum operation temperature is limited and they are also often incompatible with the elastomer compounds from which the stern tube seals are made [
6].
Poly-α-olefin (PAO) is a synthetic compound obtained from polymerizing α-olefin. PAO is non-polar in nature, so HEES and PAG, both polar, have a higher affinity to metal surfaces. Consequently, PAOs are often mixed with esters, acting as carrier of the polar additives [
7] to increase the additive solubility. There is an ongoing discussion as to whether or not PAOs are actually EALs, since they do not meet any renewable source standards and only the low viscosity types are biodegradable. In addition, they are not listed with the other three EALs in the VGP 2013 [
3]. Both the base oil and additive formulation must meet the VGP legislation so the final formulation can be labelled as an EAL; consequently, the type of additives allowed is also limited [
8].
Several EALs are readily available in the market, making the selection of a suitable lubricant rather complex. The current trend, as shown in the VGP 2018 draft, is that such environmental regulations become stricter with time. Nevertheless, EALs have caused controversy since they became mandatory. In addition to being more costly than mineral oil-based lubricants, it is thought that sailing with EALs causes a higher oil spillage while shortening the service life of stern tube seals. Regardless of the technical complexity of obtaining data on the stern tube of sailing vessels, running conditions differ from ship to ship, making it impossible to reliably compare the impacts of specific lubricants alone. Computational models for rotary lip seals are available in the literature; however, those models commonly assume Newtonian behavior of the lubricant [
9,
10]. It is unknown if the results from these models still apply when the seals operate with EALs instead, as the rheological properties are not well characterized.
For rotary lip seals, the optimal friction, leakage, and wear rate are determined during the transition from mixed to the full-film lubrication regime [
11]. For a fixed gap geometry (including roughness), load
, and velocity
on a certain contact, the lubrication regime only depends on viscosity
. The viscosity of a lubricant relates the shear stress
to the shear rate
. The viscosity of a fluid depends on the following: composition, temperature, shear rates, pressure, and time.
The importance of EAL composition is self-explanatory. The nature of both the base oil and the additive package play an important role in chemical incompatibility [
12] with the surrounding hardware and harmful fluids (i.e., water). The possible ingress of water into the stern tube would lead to an emulsion that would affect the original properties of the lubricant. The impact of water on EALs is beyond the scope of this paper. It is difficult to break apart the formulation of a lubricant; however, the presence (or lack) of certain components can be identified using Fourier transform infrared (FT-IR) or Raman spectroscopy [
13].
Lubricant formulations often use viscosity index modifiers (VII), which increase the overall viscosity of the base-lubricant blend [
8]. These are usually long polymeric chains that help transmit tangential loads between the different lubricant layers. As a result of high loading, high temperature, or long running periods, the rupture of those VIIs can lead to a permanent loss of viscosity. Even if the polymeric chains show a high shear stability, they can be oriented in the direction of the flow due to shear stress, leading to a temporary loss of viscosity. In extreme situations (at higher shear rates), the lubricant takes the viscosity of its base oil or blend of base oils [
7].
The viscosity index (VI) is a measure of the viscosity dependence on temperature
. Temperature-viscosity data are usually provided by the lubricant supplier. The shear stress of mineral oil-based lubricants is generally considered to be directly proportional to the shear rate applied to it (i.e., Newtonian behavior). However, when additives (e.g., VIIs) and contaminants increase the viscosity of the fluid at low shear rates, the lubricant can become susceptible to shear thinning. In stern tube seals, the shear rate of the lubricant is high. The ratio between the shaft liner velocity and the oil film thickness on the elastomeric seals leads to shear rates up to 10
6 s
−1. Shear thinning can be measured via specialized rheometers that allow reaching such high shear rates. The pressure-viscosity coefficient α relates the increase in the viscosity of a lubricant with pressure. Neither the pressure on the stern tube chamber nor the hydrodynamic pressure between the stern tube seals and the liner exceed 10 MPa [
14]. It can be estimated that the impact of pressure on the lubricant viscosity, even with the highest pressure-viscosity coefficients, is relatively small and can be thereby disregarded in stern tube seals.
The capability of a liquid to spread on a surface is known as wettability. Wettability cannot be disregarded in the case of stern tube seals since it determines if the fluid enters the contact zone. The affinity of the lubricant to the surrounding surfaces is a system variable and therefore it is not exclusively dependent on the fluid but also on the contacting surfaces [
15]. Properties like foaming depend on viscosity and surface tension of the lubricant [
7]. Both chemical incompatibilities and rheological differences with a mineral based-oil can cause EALs to perform differently.
This paper focuses on the viscosity and wettability of the lubricant, as these factors determine the properties for the lubrication and sealing performance. The viscosity of new Environmentally Acceptable Lubricants under the high shear rates found in the stern tube applications has been well characterized. In detail, a set of EALs are compared to a mineral oil-based lubricant in terms of viscosity, density, and wetting characteristics under demanding conditions. Rheological models are fitted to the measurements so these can be used in more complex lubrication models.
2. Materials and Methods
One traditional mineral oil-based marine lubricant as well as five readily available EALs were selected and are listed in
Table 1. Each lubricant contains a base oil (or a blend) mixed with an additive package. Lubricant 1 is not an EAL. Lubricants 2 and 3 are synthetic esters from the same supplier as Lubricant 1. Lubricants 4 and 5 are PAOs from a second supplier and Lubricant 6 is a synthetic ester obtained from a third supplier.
The lubricants tested are used in the stern tube lubrication of big vessels. Typical operation conditions of the lubricant placed between the seals and the shaft liner (
Figure 1) are shown in
Table 2.
Under operational conditions, the stern tube lubricant temperature is strongly related to the sea water temperature. The ship shaft acts as a heat pipe releasing most of the heat dissipated in the stern tube bearings and seals. However, the temperature close to the running surface between the seals and liner can exceed 100 °C at the highest shaft angular speeds. A wide range of shear rates can occur depending on the operational conditions in different stern tubes. Consequently, different viscometers were used to cover a wide range of shear rates. The measurements at different temperatures and low shear rates were carried out using a low-shear rate viscometer Brookfield Pro+II (Brookfield, Middleboro, MA, USA) shown in
Figure 2a. An Ultra Shear Viscometer (PCS Instruments, London, UK) was used to reach shear rates between 10
6 and 10
7 s
−1 (
Figure 2b). To find out if a permanent loss of viscosity developed in the lubricant, the measurement at 10
6 s
−1 was repeated after testing a sample at the highest shear rates. The average of five measurements was calculated at each operational condition (temperature and shear rate). The high shear rate viscometer typically achieved a repeatability of 2% of the viscosity value.
The density of each lubricant was measured using a high precision Stabinger viscometer SVM 3000 (Anton Paar, Graz, Austria), which additionally works as a densitometer. The device accurately measures the viscosity at low shear rates, so the measurements obtained with the Brookfield viscometer could be validated.
The Wilhelmy plate method on a DCAT11 tensiometer (DataPhysics, Filderstadt, Germany) was used to measure the surface tension
of the different lubricants (
Figure 3). A platinum plate was immersed on the lubricant sample and the necessary load to pull it out was recorded. Once the plate properties and the length of the wetted perimeter of the plate
were known, it was possible to deduce the surface tension of the liquid from that pulling load
, as shown in Equation (1). The lubricants tested showed a good wettability on metal surfaces with contact angles below 10° and hence complete wetting can be assumed (
).
The test was repeated three times per lubricant and the plate was thoroughly cleaned each time with a fire gun.
All the lubricants were analysed using a Spectrum 100 FT-IR Spectrometer (PerkinElmer, Waltham, MA, USA) with attenuated total reflection (ATR) sampling technique. Although the FT-IR technique does not reveal the specific formulation of each lubricant, the main components of the different lubricants can be identified and compared.
Gel Permeation Chromatography (GPC) was used to measure the molecular weight distribution of the different lubricants. The device used was an Agilent 1200 series (Agilent, Santa Clara, CA, USA) with a refractive index and an ultraviolet (UV) detector at 254 nm with three GPC PLgel 3 μm MIXED-E columns in series. The column was operated at 23 °C with tetrahydrofuran as solvent with a flowrate of 1 mL/min. Polystyrene solutions ranging from 162 to 27,810 Da were used to calibrate the device.
4. Discussion
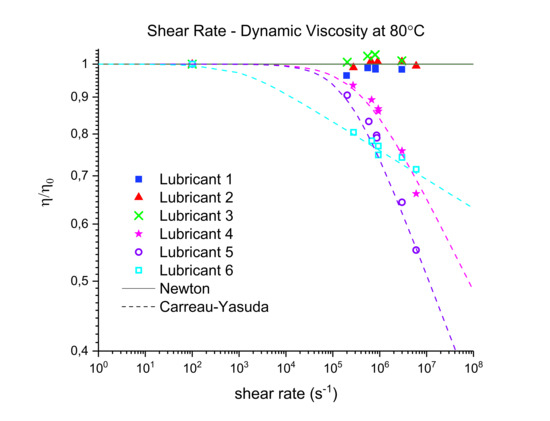
Three out of five EALs tested showed shear thinning at shear rates above 105 s−1. Shear thinning was observed in both synthetic ester as well as poly-α-olefin-based EALs. Whether or not shear thinning occurs in a stern tube depends, to a large extent, on the operational conditions in the stern tube. Assuming stern tube seals operate under their optimal point, i.e., lowest friction and wear rate values, the lubricant film thickness must be around the average roughness of the liner surface. With this educated guess, we deduced that the shear rate operational values of a ship fall within the range where shear thinning was observed.
It has been suggested that the presence of shear thinning is related to the amount and type of viscosity index improvers (VIIs) used within the lubricants formulation [
24]. The IR spectra of Lubricants 2, 3, and 6 were similar; however, Lubricant 6 shear thinned at the shear rates tested whereas 2 and 3 did not. The shear thinning curves in
Figure 7 show a completely different shear thinning development for the ester- and PAO-based lubricants. The PAO-based lubricants (4 and 5) presented a sudden decrease in viscosity beyond a certain critical shear rate; the viscosity of lubricant 6 started at a lower shear rate and experienced a steady viscosity decrease. The shape of the polymer chains and intermolecular forces determine their ability to stretch and align themselves in the direction of flow causing a decrease in viscosity. Therefore, the different lubricant responses to shear stress shown in
Figure 7 are a result of the shape, size, and interactions of the VIIs used in their formulations. A permanent loss in viscosity is attributed to the scission of the larger hydrocarbon chains of the VIIs due to mechanical loading. Lubricant 6 showed a certain polymer-cracking by shear as a result of being tested at 10
7 s
−1 (
Table 6).
The measured surface tension was shown to be within the same range for all the lubricants tested, independent of the nature of the base oil used in its formulation. The polarity of a synthetic ester is determined by the alcohols and carboxylic acids used in their formulation. Pure poly-α-olefins have a surface tension around 20 mN/m [
25], which is within the range of the final PAO-based lubricants measured. The use of certain additives, like foam inhibitors, act as surface tension depressants and could mask the surface tension of the final lubricants [
7]. Surface tension depressants are also commonly used to decrease the amount of swelling or shrinkage of elastomers immersed in hydrocarbon fluids [
7], which is an undesirable effect in dynamic seals.
FT-IR analysis revealed that all the EALs tested contained ester-based components. Synthetic esters are frequently used in PAO-based formulations as additives carriers due to their more-polar nature [
7]. Consequently, the PAO-based EALs tested can be susceptible to hydrolytic degradation due the certain amount of present esters. Lubricants 2 and 3 are synthetic esters-based from the same manufacturer. Although they have different viscosities, they displayed the same IR spectra because the functional groups are the same. The same phenomenon occurred with Lubricants 4 and 5. The GPC allowed us to distinguish the lubricants from the same manufacturer. The blend of base oils became clear for both the synthetic esters- and the PAO-based lubricants. The amount of higher molecular weight components could explain the higher viscosity of Lubricant 3 with respect to Lubricant 2. This was not as evident for the PAO-based lubricants (Lubricants 4 and 5); however, the degree of branching of different oligomers and the affinity with the solvents also plays a role in the overall viscosity. As mentioned, by polystyrene calibration standards, polymeric chains that significantly contribute to the rheology of the lubricant might unfortunately be ignored. In other words, the GPC mainly covered low molecular weight lubricant compounds. No correlation between the molecular weight distribution and the shear thinning could be established because the high molecular weight range that could confirm the presence of VIIs was neglected.
From this research, we concluded that EALs cannot directly be treated as common mineral oil-based lubricants. Although EALs perform fairly similarly with temperature as the traditional stern tube mineral oils, at high shear rates, a viscosity drop is observed for certain EALs (i.e., shear thinning). Under the same high shear running conditions, the thickness of the film between the seals and the shaft liner will be reduced when using certain EALs. By running at lower viscosities, an increase in temperature can occur if the mixed-lubrication regime is entered. Although shear thinning is not a negative characteristic per se, if the viscosity drops below a certain value, higher wear rates, temperatures, and friction loads are expected, which would consequently shorten the lifespan of the stern tube seals.
Rheological models were fitted to the measurements and accurately predicted the density, viscosity and surface tension of these lubricants for conditions as they occur in stern tube seals. Therfore, it is now possible to predict the impact of EALs in more complex mechanical models.