1. Introduction
This study investigates the relationship between the composition of a secondary structure (SS), formed on the friction surface of steel after wear tests against experimental aluminum bearing alloys and steel wear resistance.
Many works have been published regarding the SSs formed on the various friction surface. In addition, almost all of these studies began in earnest well over 100 years ago [
1,
2]. The paper [
3] shows the value of a transfer film that was formed as a consequence of the interaction of the lubricating media with the metal surface. In works of other authors [
4,
5,
6,
7,
8,
9], the importance of tribofilms on the surface in boundary lubrication has been determined.
Various friction surface characteristics have been investigated: geometric parameters (roughness, curvature, asperities), the microstructure and plastic deformation of tribosurface layers after friction (metal surface layer formation [
10]), the size and microhardness of secondary structures, elemental and chemical compositions, various aspects of chemical and physical bonds, the distribution of elements and chemical compositions, etc.
A large number of investigations of secondary structures on worn surfaces due to the formation of tribolayers during rubbing have been carried out because this layer can significantly influence the tribological behavior of the system.
Previous investigations have shown that the formation of SSs is accompanied by a negative production of entropy and consequently leads to a decrease in the intensity of wear, which was confirmed by running-in results [
11,
12,
13,
14,
15,
16]. During the running-in stage, not only an increase in the contact area and a decrease in the contact load but also the formation of secondary structures occurs. Furthermore, it has been shown [
17] that the elemental composition of the SSs was formed at the initial stage of friction.
Thus, there is great and long-term interest in secondary structures due to the fact that these structures lead to a decrease in the intensity of wear.
Despite many investigations of SSs for various tribosystems and friction conditions and for different materials, lubricants, and media, the findings of such studies are not presented. In other words, there are no recommendations or modification principles for rubbing materials, media, or lubricants to facilitate the formation of SSs.
The process of friction is an artificial process, and the response of the tribosystem to friction is a natural process. Similar to the Le Chatelier-Brown principle, it should be noted that, in order to decrease external intervention (friction), the tribosystem should respond to it. The important consequence of friction is wear. Thus, the response of the tribosystem to friction is the formation of secondary structures, which contribute to the decrease in wear. We assume that a tribosystem can tune themselves, i.e., to find the optimized composition of secondary structures within its capacity. The origin of constituents of contacting bodies, media, and lubricants can be elements of secondary structures. In order to determine chemical elements that are used for the formation of secondary structures, the studies of these structures have to be conducted. This knowledge base enables us to provide recommendations on how the change in the chemical composition of the present tribocouple can improve the formation of secondary structures.
Therefore, the aim of this work is not as much to describe structure or the formation of secondary structures as it is to detect elemental composition and their influence on the intensity of wear.
In contrast to our previous research, where the secondary structures on the friction surface of aluminum-based antifriction materials were studied [
18,
19,
20], the present research is focused on the secondary structures on the steel counterbody.
2. Materials and Methods
The surface of structural high alloy chromium-nickel steel rollers (38CrNi3Mo closest equivalent SNC28, Hakuro Group, Kasai, Japan) after friction tests against experimental cast aluminum alloys was the object of the study. Steel specimens were extracted from the actual shafts of diesel locomotive engines. The hardness of the steel was 269 HB. The initial chemical composition of steel and alloy samples before friction was detected by emission spectrometer Spectrolab-S (Spectro Analytical Instruments GmbH, Kleve, Germany) and is shown in
Table 1 and
Table 2, respectively.
The composition of experimental aluminum antifriction alloys for monometallic journal bearings have an Sn content between 8.7% and 11% (medium-tin alloys) and between 5.4% and 7.6% (low-tin alloys). Additionally, these alloys have a lead, copper, silicon, magnesium, and zinc in its chemical composition (
Table 2).
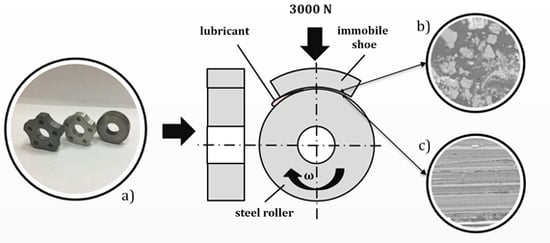
Wear tests were carried out using a friction machine “SMC-2” (Ziptest Ltd., Ivanovo, Russia) and according to the rotating steel roller-fixed shoe of aluminum alloy scheme (
Figure 1). Roller diameter was 40 mm and width was 10 mm. The working surface of the aluminum alloy shoe was a 10 mm width and a curvature radius of 20 mm. The samples were tested for 40 h under lubricated boundary friction conditions with a constant load of 617 N.
Mineral single grade motor oil M14V2 belongs to the group of API CB was used as a lubricant. Mass fraction of active elements in motor oil M14V2 is provided in
Table 3. The oil was supplied to the friction area dropwise at a rate of 0.2 g/min. The rotational frequency of the rollers was 500 rev/min.
Temperature on the surface of the roller was measured with a pyrometer Testo 830-T2 (Testo SE & Co, Lenzkirch, Germany). After the wear test, rollers were degreased, washed, and dried. Each roller was weighed using a GR-300 (A&D Weighing, Tokyo, Japan) electronic analytical balance of a 0.0001 g accuracy.
The study of the microstructure and chemical analysis of the surface of steel samples after friction was carried out in scanning electron microscope (SEM) “Vega 3 LMH” (Tescan, Brno, Czech Republic), equipped with an Oxford Instruments (Abingdon, UK) energy-dispersive analysis (EDX) module.
Each specimen was examined at three magnifications, 100×, 500×, and 5000×, for studying areas of different size to detect the chemical composition changes compared to the initial state. Prior to SEM observations, after the friction tests, the steel samples were washed with warm water, degreased with carbon tetrachloride, kerosene, and acetone, and then washed again with cold water.
3. Results and Discussion
The values of friction coefficient, the temperature on the surface of the steel roller at the exit from the friction zone, and the wear weight loss of the steel obtained after wear tests are presented in
Table 4.
These results demonstrate that there is no correlation between steel wear, friction coefficient, and roller surface temperature, i.e. surface temperature and wear do not necessarily decrease with a decrease in friction coefficient. This may be due to the processes occurring in the SS [
21]. Additionally, there was no apparent connection between the content of the soft structural component of the alloy (mainly tin) and steel wear. Steel rollers, which worked in tandem with Alloys 1 and 2 with the highest tin content and with Alloys 7 and 8 with the lowest tin content, have less wear (
Table 1). Therefore, in this range of aluminum alloy compositions, their basic composition does not have a decisive influence on the wear of the steel counterbody. These results allow us to suggest that the elements that are mainly transferred to the SS from the steel can result in significant changes in steel wear rates.
The results of microstructural and energy dispersive analyses of the contact area of the steel exhibit a non-uniform striated surface structure with sticking and leveling of different size, and the presence of the constituents of the aluminum alloy and the lubricant on the friction surface.
Figure 2 shows a typical microstructure of rubbed steel surface coupled with an AO-5.8 aluminum alloy.
Though all the steel specimens were of the same initial composition, chemically different secondary structures were formed depending on the grade of the counterpart alloy. Secondary structures include all of the chemical elements in the tribosystem, which is a consequence of its self-organization. Generally, SSs are thin metal-polymer films generated as a result of the high carbon and oxygen content. The presence of a metal-polymer film on the steel surface was clearly demonstrated by investigation of an SEM image (
Figure 3A) in which the film was torn off and rolled into a bundle.
Most typical compositions of specific areas of the worn steel surface are given in
Table 5, proving that the secondary structure film mainly consists of carbon and oxygen. However, the film is so thin that, in places where it is located in a single layer, the analysis fixes the advantage of the metal components of the alloy over the polymer base of the film. The film is transparent to the electron microscope. Film thickness does not exceed 1 micron.
Figure 3D,E show the distribution maps of molybdenum and sulfur. It is necessary to note that S and Mo have identical distribution. Such dependence can be traced on almost all tested steel surfaces. This suggests the possibility of the formation of molybdenum disulfide in the secondary structures, which has a positive effect on tribological properties.
In order to compare elemental composition of pristine and worn steel surfaces, the EDX technique was used. The average chemical composition of the steel surface before friction is shown in
Table 6. This composition is considered the starting point for comparison with the composition of SS formation on the steel surface after wear tests.
Table 7 exhibits EDX analysis of elements of secondary structures for the steel roller tested against all studied aluminum alloys. The results show that the formed secondary structures are characterized by an increase in carbon and oxygen content. Additionally, the presence of chlorine and fluorine was detected, and these elements were released from the lubricant. In addition, there was a mass transfer of aluminum to the steel friction surface.
The iron content of the SS is lower than that of the wear surface before friction by approximately 10%. However, the concentration of Ni, Cr, Mg, Si, and Mo remains at the same level proportional to the reduction of the Fe content.
This observation indicates the presence of the additional mass transfer of these elements to the friction surface. The content of molybdenum in secondary structures increased markedly. In this manner, a higher content of Ni, Cr, Mn, Si, and Mo on the wear surface was found in comparison with that on the pristine steel surface (
Table 4). Besides the existence of O, C, and Al, the presence of high content of F was observed. Additionally, an almost full absence (<0.1%) of the soft structural components (Sn and Pb) of the antifriction materials should be noted. Contrary to the popular wisdom that spreading the soft structural component (Sn–Pb) on a steel surface improves wear resistance, a slight effect of a mass transfer of Pb and Sn from the antifriction material to the steel surface was observed [
22]. This was confirmed by the fact that alloys with a low-tin content (No. 5–8,
Table 4) cause a lower wear rate of steel than alloys with a medium-tin (No. 1–4,
Table 4) content, although it is generally believed that the opposite is true.
Table 4 and
Table 7 show the positive influences of Mo in the SS on the wear of steel.
Table 4 indicate an absence of Mo in the secondary structures of steel against Alloy 3, while in the SS of steel rubbed against Alloy 6 the content of Mo is only 0.3%. In the rest of the SSs, the content of Mo is about 0.5–0.6%. The highest and the second largest wear rate was observed in the tribocouples of Steel-Alloys 3 and 6, respectively (
Figure 4A). That the maximum wear of steel corresponds to a zero molybdenum content in secondary structures and that, as the content of molybdenum increases, wear decreases is clear confirmation of the beneficial effect of molybdenum on the wear resistance of steel samples. SEM-EDS and Spectrolab analysis of the unworn surface of steel revealed a concentration of Mo of about 0.4% and 0.48%, respectively (
Table 1 and
Table 4). Thus, the Mo content was increased by a factor of 1.5, compared to the content before friction (
Table 7).
The effect of molybdenum on the friction coefficient (
Figure 4B) can be described by a nonlinear dependence. The friction coefficient decreased from 0.019 to 0.014 with 0.3% Mo content in secondary structures on the friction surface of steel samples. As the Mo content further increased the friction coefficient increased as well.
The presence of fluorine in secondary structures on the worn steel roller surface tested against aluminum alloys was observed in all studied tribopairs. The positive influence of F in SSs on the wear of steel can be seen from a comparison of
Table 4 and
Table 7, although this influence is not as straightforward as in the case of Mo. From the graph (
Figure 5A) it can be seen that the increase in the fluorine content in the secondary structures of steel samples has a positive effect on the wear resistance of steel. On the one hand, the low level of F in SSs and the highest wear of steel were found in the tribosystems of Steel-Alloys No. 3 and No. 6. The highest concentration of F in SSs and the lowest wear rate of steel were observed in the tribopairs of Steel-Alloys 1, 7, and 8. In the meantime, the lowest level of F in SSs of steel was detected in Steel-Alloy 2. Meanwhile, a high level of F in SSs and a moderate wear rate of steel were found in the tribosystems of Steel-Alloys 4 and 5.
The tendency for the coefficient of friction to increase with an increase in the content of fluorine in steel is most pronounced in the interaction of steel with medium-tin alloys (
Figure 5B). For them, the coefficient of friction is as follows: 0.015 (2), 0.019 (3), 0.020 (1), and 0.022 (4), with a range of concentrations of fluorine from 0.9 to 1.6% F. When working with low-tin alloys, except for the AO 7.6 (5) alloy, whose friction coefficient falls out of all the laws, the values are 0.014 (6), 0.018 (7), and 0.017 (5), which correspond to the curve with a maximum. In general, it can be claimed that fluorine contained in the secondary structures of the friction surfaces of steel specimens contributes to the growth of friction coefficient.
Steel does not contain F. However, in the present tribosystems, only aluminum-based antifriction alloys contain about 0.01% fluorine. Nevertheless, the concentration of F in secondary structures was found to be two orders of magnitude higher than the one measured in the tribosystem. These results allow us to suggest that the presence of F is essential in SSs to reduce wear of steel.
The highest content of Cr in the SS of steel was found in the tribosystems of Steel-Alloys 1, 5, and 7 among all studied samples (
Table 7). The steel counterbody in the tribosystems of Steel-Alloys 1 and 7 has the lowest wear rate, as compared to the rest of the tribopairs. The only exception is a steel counterbody in the tribocouple of Steel-Alloy 5. In this particulate case, the level of Cr in the SS is 0.9%, and a moderate wear rate was observed. The high concentration of Cr in the SS as compared with the concentration of the unworn steel surface allows us to assume that the presence of Cr is essential in SSs to reduce wear of steel.
A strong relationship between Ni content in SSs and wear rate was not detected. For instance, a higher level of Ni was found in secondary steel structures tested against Alloys 3 and 7. These steel counterbodies showed the highest and lowest wear rates, respectively (
Table 7). However, the percentage increase of Ni concentration in the SS reported as 10% and 15% as compared to the concentration of the unworn steel surface allows us to assume that the presence of Ni is essential in SSs to reduce wear of steel.
Figure 6 shows distribution maps of elements of secondary structures. Each observed area contained oxygen, which is a part of a metal-polymer film. Local spectrum analysis revealed that areas with the highest iron content do not contain oxygen (<0.1%) but do contain fluorine (1.5% F). Oxygen was not detected in the fluorine-rich areas (
Figure 6C,D). Fluorine distribution maps in most cases coincide with iron ones (
Figure 6D,E). However, in the zones enriched with aluminum and oxygen, content of fluorine decreases. Additionally, the presence of fluorine reduces the importance of carbon and oxygen, having little effect on the metallic ingredients of secondary structures.
A strong relationship between O, C, Mn, Al, and Si content and wear rate was not detected. However, taking into account the higher level of these elements in SSs as compared to the concentration of the unworn steel surface allows us to assume that the presence of these elements is essential in SSs to reduce wear of steel as well.
The low level of Al in the SS of steel (0.1–0.4%) allows us to suggest that friction was without a seizure and that the lubricant film was not ruptured.
4. Conclusions
SSs on the steel surface before and after friction against Al-based alloys with various concentrations of Sn were studied.
A relationship between wear rate, surface temperature, the friction coefficient of the steel counterbody, and the concentration of the soft structural component of the antifriction alloy was not found.
Secondary structures may contain a transparent polymeric film (up to 1 µm thick) with metal inclusions. This film may be disrupted or even slip and thereby may be formed into a roll of thin polymeric coating.
Below this film, there is another type of secondary structure, which is the steel surface with mass-transferred alloying elements of steel: Mo, Cr, Ni, Si, and Mn. Some elements were transferred onto the steel surface from oil, an ambient atmosphere, and an aluminum counterbody: S, F, Al, C, and O. The concentration of all these elements in SSs is higher compared with the concentration of the unworn steel surface. Meanwhile, some elements (Sn, Pb, and Mg) were not influenced by the friction.
Elements of SSs interact actively with one another. Therefore, areas rich in Mo reacted with S and perhaps formed a wear-resistant MoS2-based structure similar to the structure on the antifriction alloy. The wear rate of the steel counterbody decreased as the concentration of Mo in SSs decreased.
The presence of F, Cr, and Ni in the steel SSs reduced the wear rate, although this presence is not as straightforward as in the case of Mo.
The low level of Al and the absence of Sn in steel SSs is also worth mentioning.
The correlation between the concentration of O and C, and steel wear rate was not found.
As outlined above, the formation of SSs reduces the steel wear rate, so a higher concentration of Mo, Cr, Ni, and Si in steel or the counterbody is favorable for the formation of SSs with optimal composition. The addition of F and S to a lubricant composition can be recommended for the same reasons.
Since the formation of secondary structures is favorable, complex alloy steels should be used to reduce steel wear rate.