Absorption Wavebands for Discriminating Oxidation Time of Engine Oil as Detected by FT-IR Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
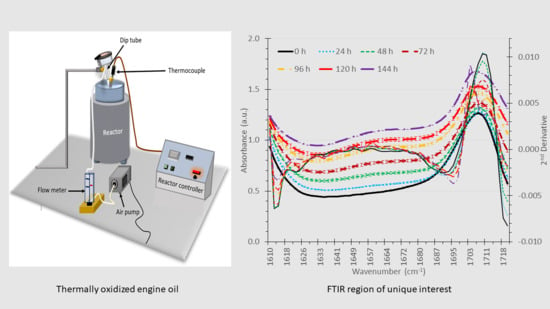
2.1. Thermal Oxidation
2.2. FT-IR Analysis
2.3. Data Preprocessing and Analysis
3. Results and Discussion
3.1. Selection of Wavenumbers and Wavenumber Ranges
3.2. Predicting Oxidation Time
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Macián, V.; Tormos, B.; Gómez, Y.A.; Salavert, J.M. Proposal of an FTIR Methodology to Monitor Oxidation Level in Used Engine Oils: Effects of Thermal Degradation and Fuel Dilution. Tribol. Trans. 2012, 55, 872–882. [Google Scholar] [CrossRef] [Green Version]
- Soleimani, M.; Sophocleous, M.; Glanc, M.; Atkinson, J.; Wang, L.; Wood, R.J.K.; Taylor, R.I. Engine oil acidity detection using solid state ion selective electrodes. Tribol. Int. 2013, 65, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Faure, D.; Hipeaux, J.C.; Guevellou, Y.; Legros, A. Oxidation stability of gasoline engine lubricants: Effect of base-oil chemistry in laboratory and engine tests. Lubr. Sci. 1999, 5, 337–360. [Google Scholar] [CrossRef]
- Lansdown, A.R. Lubrication and Lubricant Selection a Practical Guide, 3rd ed.; The American Society of Mechanical Engineers: New York, NY, USA, 2004. [Google Scholar]
- Aikawa, K.; Maruyama, M. Development of an Oil Deterioration Monitoring System by Estimating Base Number; SAE Technical Paper 2007-01-1565; SAE International: Warrendale, PA, USA, 2007. [Google Scholar] [CrossRef]
- Soleimani, M.; Sophocleous, M.; Wang, L.; Atkinson, J.; Hosier, I.L.; Vaughan, A.S.; Taylor, R.I.; Wood, R.J. Base oil oxidation detection using novel chemical sensors and impedance spectroscopy measurements. Sens. Actuators B Chem. 2014, 199, 247–258. [Google Scholar] [CrossRef] [Green Version]
- Amat, S.; Braham, Z.; Le Dreau, Y.; Kister, J.; Dupuy, N. Simulated aging of lubricant oils by chemometric treatment of infrared spectra: Potential antioxidant properties of sulfur structures. Talanta 2013, 107, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Canter, N. Use of antioxidants in automotive lubricants. Tribol. Lubr. Technol. 2008, 64, 12–19. [Google Scholar]
- Basu, A.; Berndorfer, A.; Buelna, C.; Campbell, J.; Ismail, K.; Lin, Y.; Rodriguez, L.; Wang, S.S. Smart Sensing of Oil Degradation and Oil Level Measurements in Gasoline Engines; SAE Technical Paper 2000-01-1366; SAE International: Warrendale, PA, USA, 2000. [Google Scholar] [CrossRef]
- ASTM. ASTM D2896—15 Standard Test Method for Base Number of Petroleum Products by Potentiometric Perchloric Acid Titration; ASTM: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Hu, T.; Teng, H.; Luo, X.; Chen, B. Impact of Fuel Injection on Dilution of Engine Crankcase Oil for Turbocharged Gasoline Direct-Injection Engines. SAE Int. J. Engines 2015, 8, 1107–1116. [Google Scholar] [CrossRef]
- Agoston, A.; Otsch, C.; Jakoby, B. Viscosity sensors for engine oil condition monitoring—Application and interpretation of results. Sens. Actuators A Phys. 2005, 121, 327–332. [Google Scholar] [CrossRef]
- Cheek, G.T.; Mowery, R. Determination of Antioxidants in Lubricating Oils Using Ultramicroelectrodes. Anal. Chem. 1989, 61, 1467–1469. [Google Scholar] [CrossRef]
- Dickert, F.L.; Forth, P.; Lieberzeit, P.A.; Voigt, G. Quality control of automotive engine oils with mass-sensitive chemical sensors—QCMs and molecularly imprinted polymers. Fresenius’ J. Anal. Chem. 2000, 366, 802–806. [Google Scholar] [CrossRef]
- Duchowski, J.K.; Mannebach, H. A Novel Approach to Predictive Maintenance: A Portable, Multi-Component MEMS Sensor for On-Line Monitoring of Fluid Condition in Hydraulic and Lubricating Systems. Tribol. Trans. 2006, 49, 545–553. [Google Scholar] [CrossRef]
- Kauffman, R.E. Development of a Remaining Useful Life of a Lubricant Evaluation Technique. Part Ill: Cyclic Voltammetric Methods. Lubr. Eng. 1989, 45, 709–716. [Google Scholar]
- Lieberzeit, P.A.; Glanznig, G.; Leidl, A.; Voight, G.; Dickert, F.L. Nanostructured Polymers for Detecting Chemical Changes During Engine Oil Degradation. IEEE Sens. J. 2006, 6, 529–535. [Google Scholar] [CrossRef]
- Moon, S.-I.; Paek, K.-K.; Lee, Y.-H.; Kim, J.-K.; Kim, S.-W.; Ju, B.-K. Multiwall Carbon Nanotube Sensor for Monitoring Engine Oil Degradation. Electrochem. Solid-State Lett. 2006, 9, H78–H80. [Google Scholar] [CrossRef]
- Price, R.J.; Clarke, L.J. Chemical Sensing of Amine Antioxidants in Turbine Lubricants. Analyst 1991, 116, 1121–1123. [Google Scholar] [CrossRef]
- Smiechowski, M.F.; Lvovich, V.F. Iridium oxide sensors for acidity and basicity detection in industrial lubricants. Sens. Actuators B Chem. 2003, 96, 261–267. [Google Scholar] [CrossRef]
- Méndez Aller, M.; Abdul-Munaim, A.M.; Watson, D.G.; Preu, S. Error Sources and Distinctness of Materials Parameters Obtained by THz-Time Domain Spectroscopy Using an Example of Oxidized Engine Oil. Sensors 2018, 18, 2087. [Google Scholar] [CrossRef]
- Ahmad, I.; Ullah, J.; Ishaq, M.; Khan, H.; Gul, K.; Siddiqui, S.; Ahmad, W. Monitoring of oxidation behavior in mineral base oil additized with biomass derived antioxidants using FT-IR spectroscopy. R. Soc. Chem. Adv. 2015, 5, 101089–101100. [Google Scholar] [CrossRef]
- Guan, L.; Feng, X.L.; Xiong, G.; Xie, J.A. Application of dielectric spectroscopy for engine lubricating oil degradation monitoring. Sens. Actuators A Phys. 2011, 168, 22–29. [Google Scholar] [CrossRef]
- Rahimi, B.; Semnani, A.; Nezamzadeh-Ejhieh, A.; Langeroodi, H.S.; Davood, M.H. Monitoring of the Physical and Chemical Properties of a Gasoline Engine Oil during Its Usage. J. Anal. Methods Chem. 2012, 2012, 819524. [Google Scholar] [CrossRef]
- van de Voort, F.R.; Sedman, J.; Cocciardi, R.A.; Pinchuk, D. FTIR Condition Monitoring of In-Service Lubricants: Ongoing Developments and Future Perspectives. Tribol. Trans. 2006, 49, 410–418. [Google Scholar] [CrossRef]
- Ofunne, G.C.; Maduako, A.U.; Ojinnaka, C.M. Studies on the ageing characteristics of automotive crankcase oils. Tribol. Int. 1989, 22, 401–404. [Google Scholar] [CrossRef]
- Ofunne, G.C.; Maduako, A.U.; Ojinnaka, C.M. High temperature oxidation stability of automotive crankcase oils and their base oils. Tribol. Int. 1990, 23, 407–412. [Google Scholar] [CrossRef]
- ASTM International. ASTM E2412-10(2018) Standard Practice for Condition Monitoring of In-Service Lubricants by Trend Analysis Using Fourier Transform Infrared (FT-IR) Spectroscopy; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar] [CrossRef]
- Egharevba, F.; Maduako, A.U. Assessment of oxidation in automotive crankcase lube oil: Effects of metal and water activity. Ind. Eng. Chem. Res. 2002, 41, 3473–3481. [Google Scholar] [CrossRef]
- Nguele, R.; Al-Salim, H.; Sasaki, K. Oil Condition Monitoring Degradation Mechanisms and Additive Depletion. J. Multidiscip. Eng. Sci. Technol. 2015, 2, 355–360. [Google Scholar]
- Moehle, W.E.; Cobb, T.W.; Schneller, E.R.; Gatto, V.J. Utilizing the TEOST MHT® to Evaluate Fundamental Oxidation Processes in Low-Phosphorus Engine Oils. Tribol. Trans. 2007, 50, 96–103. [Google Scholar] [CrossRef]
- Nie, B.; Stutzman, J.; Xie, A. A vibrational spectral maker for probing the hydrogen-bonding status of protonated Asp and Glu residues. Biophys. J. 2005, 88, 2833–2847. [Google Scholar] [CrossRef] [PubMed]
- As’ad, A.M.; Yeneneh, A.M.; Obanijesu, E.O. Solvent Dewaxing of Heavy Crude Oil with Methyl Ethyl Ketone. J. Pet. Environ. Biotechnol. 2015, 6, 213. [Google Scholar] [CrossRef]
- Kalyani; Jaiswal, V.; Rastogi, R.B.; Kumar, D. Tribological investigations on β-lactam cephalosporin antibiotics as efficient ashless antiwear additives with low SAPS and their theoretical studies. RSC Adv. 2014, 4, 30500–30510. [Google Scholar] [CrossRef]
- Yao, T.; Yang, H.; Guo, L.; Fei, Y.; Jiang, H.; Bian, S.; Wu, T. The Deterioration Mechanism of Diester Aero Lubricating Oil at High Temperature. J. Spectrosc. 2017. [Google Scholar] [CrossRef]








| Wavenumber Range (cm−1) | Indicator | Wavenumber(s) Source |
|---|---|---|
| 830–880 * | Antioxidants | 850 [26] |
| 900–1000 | Antioxidants | [27] |
| 930–1020 | ZDDP Depletion | [28] |
| 950–1040 | Antioxidants | [29] |
| 960–1025 | Antiwear components | [28]; 1000 [26] |
| 1025–1075 * | Sulfonate detergents | 1054 [29] |
| 1065–1074 ** | Current study per Fishers’s LSD | |
| 1078–1144 ** | Current study per Fishers’s LSD | |
| 1100–1200 | Sulfur oxides, viscosity improvers, Sulfonate detergents, Sulfate by-products | [30]; 1116 [29]; 1180 [29]; 1120–1180 [28] |
| 1120–1140 ** | Current study per Tukey’s HSD | |
| 1178–1219 ** | Current study per Fishers’s LSD | |
| 1580–1680 * | Nitration, Olefinic absorption | 1600 [29]; 1630 [28,30] |
| 1600–1650 | Nitration | [28] |
| 1612–1668 | Current study per Fishers’s LSD | |
| 1614–1678 | Current study per Tukey’s HSD | |
| 1630–1765 * | Carbonyl compounds | 1700 [26]; 1710 [29] |
| 1650–1820 | Carbonyl compounds | [31] |
| 1660–1710 | Oxidation for ester based oils | 1660–1710 [28] |
| 1660–1800 | Oxidation | [28]; 1700 [29] |
| 1685–1725 | Oxidation | [28] |
| 1700–1750 | Carbonyl compounds | [30] |
| 1700–1800 | Carbonyl compounds | [27,29] |
| 1711–1730 ** | Current study per Fishers’s LSD | |
| 1714–1726 ** | Current study per Tukey’s HSD | |
| 3280–3580 * | Hydroxyl | 3400 [26,27,29] |
| Parameter * | Thermal Oxidation Time | ||||||
|---|---|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | 72 h | 96 h | 120 h | 144 h | |
| TBN (mg KOH/g) | 7.2 | 6.4 | 5.0 | 4.5 | 3.9 | 3.4 | 1.8 |
| Viscosity (mm2/s) at 40 °C | 44.98 | 47.67 | 46.04 | 49.38 | 49.68 | 49.25 | 53.27 |
| Wavenumber Range (cm−1) | R2 | RMSE |
|---|---|---|
| 1065–1074 | 0.972 | 8.20 |
| 1078–1144 | 0.984 | 6.14 |
| 1100–1200 | 0.972 | 8.17 |
| 1120–1140 | 0.986 | 5.76 |
| 1178–1219 | 0.976 | 7.58 |
| 1572–1593 | 0.983 | 6.31 |
| 1580–1680 | 0.991 | 4.54 |
| 1600–1650 | 0.989 | 5.04 |
| 1612–1682 | 0.993 | 4.18 |
| 1614–1678 | 0.993 | 4.13 |
| 1630–1765 | 0.977 | 7.44 |
| 1660–1710 | 0.976 | 7.58 |
| 1711–1730 | 0.980 | 6.91 |
| 1714–1726 | 0.983 | 6.40 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdul-Munaim, A.M.; Holland, T.; Sivakumar, P.; Watson, D.G. Absorption Wavebands for Discriminating Oxidation Time of Engine Oil as Detected by FT-IR Spectroscopy. Lubricants 2019, 7, 24. https://doi.org/10.3390/lubricants7030024
Abdul-Munaim AM, Holland T, Sivakumar P, Watson DG. Absorption Wavebands for Discriminating Oxidation Time of Engine Oil as Detected by FT-IR Spectroscopy. Lubricants. 2019; 7(3):24. https://doi.org/10.3390/lubricants7030024
Chicago/Turabian StyleAbdul-Munaim, Ali Mazin, Torrey Holland, Poopalasingam Sivakumar, and Dennis G. Watson. 2019. "Absorption Wavebands for Discriminating Oxidation Time of Engine Oil as Detected by FT-IR Spectroscopy" Lubricants 7, no. 3: 24. https://doi.org/10.3390/lubricants7030024
APA StyleAbdul-Munaim, A. M., Holland, T., Sivakumar, P., & Watson, D. G. (2019). Absorption Wavebands for Discriminating Oxidation Time of Engine Oil as Detected by FT-IR Spectroscopy. Lubricants, 7(3), 24. https://doi.org/10.3390/lubricants7030024