Triboemission of FINE and Ultrafine Aerosol Particles: A New Approach for Measurement and Accurate Quantification
Abstract
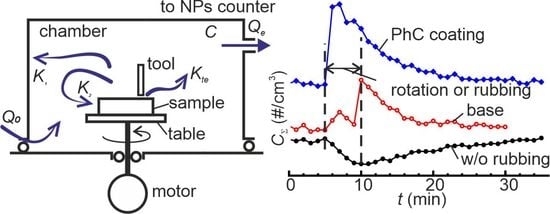
:1. Introduction
2. Model
2.1. Setup at a Standstill
2.2. Setup in Motion, without MSPE
2.3. General Solution for Particle Concentration Dynamics in an Aerosol Chamber without MSPE
2.4. Setup in Motion with MSPE
3. Experimental Setup
4. Results and Discussion
4.1. Experiments with Ambient Particle Aerosols
4.2. Dynamics of Artificial Nanoparticle Aerosols
4.3. Simulation of MSPE by Injection of Aerosols
4.4. Objective Quantification of Fine Particles Triboemission at Friction Modifier–Steel Sliding Contact
4.5. A Groundwork for the Development of the Common Standard Method and Procedure for Objective Quantification of Kinetic Parameters of Fine Particles Triboemission
- Experimental setup
- The experimental setup must have an aerosol-tight hood impeding uncontrolled dispersion of the emitted particles in and income of the particles from the environment.
- The concentration of aerosol particle in the surrounding atmosphere should be measured or controlled.
- The rotation speed and the dimensions of the moving parts must be specified.
- Experimental procedure
- A preliminary test aimed at determining the deposition velocities A1,I and A2,I of relevant aerosols must be conducted (Figure 10).
- Time series of aerosol particle concentrations must be measured before, during and after mechanical solicitation in order to determine the background particle concentrations and to measure the kinetic parameters of concentration decays.
- Data processing and reporting the results
- The rates of aerosol particles triboemission must be calculated considering the deposition velocity for each specific experimental setup and experimental conditions (rotation or linear velocity, speed profile, etc.).
- The report should include description of the geometry and dimensions of the moving parts of the setup, their rotation and linear speed, and (optionally) the deposition velocities for the relevant aerosol particles for the experimental setup.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gottschalk, F.; Sun, T.; Nowack, B. Environmental concentrations of engineered nanomaterials: Review of modeling and analytical studies. Environ. Pollut. 2013, 181, 287–300. [Google Scholar] [CrossRef]
- Wohlleben, W.; Brill, S.; Meier, M.W.; Mertler, M.; Cox, G.; Hirth, S.; von Vacano, B.; Strauss, V.; Treumann, S.; Wiench, K.; et al. On the Lifecycle of Nanocomposites: Comparing Released Fragments and their In-Vivo Hazards from Three Release Mechanisms and Four Nanocomposites. Small 2011, 7, 2384–2395. [Google Scholar] [CrossRef] [PubMed]
- Cena, L.G.; Peters, T.M. Characterization and Control of Airborne Particles Emitted During Production of Epoxy/Carbon Nanotube Nanocomposites. J. Occup. Environ. Hyg. 2011, 8, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Balyts’kyi, O.I.; Kolesnikov, V.O. Investigation of wear products of high-nitrogen manganese steels. Mater. Sci. 2009, 45, 576–581. [Google Scholar] [CrossRef]
- Kumar, P.; Pirjola, L.; Ketzel, M.; Harrison, R.M. Nanoparticle emissions from 11 non-vehicle exhaust sources—A review. Atmos. Environ. 2013, 67, 252–277. [Google Scholar] [CrossRef] [Green Version]
- Koivisto, A.J.; Jensen, A.C.Ø.; Kling, K.I.; Nørgaard, A.; Brinch, A.; Christensen, F.; Jensen, K.A. Quantitative material releases from products and articles containing manufactured nanomaterials: Towards a release library. NanoImpact 2017, 5, 119–132. [Google Scholar] [CrossRef]
- Nowack, B.; David, R.M.; Fissan, H.; Morris, H.; Shatkin, J.A.; Stintz, M.; Zepp, R.; Brouwer, D. Potential release scenarios for carbon nanotubes used in composites. Environ. Int. 2013, 59, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Vorbau, M.; Hillemann, L.; Stintz, M. Method for the characterization of the abrasion induced nanoparticle release into air from surface coatings. J. Aerosol Sci. 2009, 40, 209–217. [Google Scholar] [CrossRef]
- Koponen, I.K.; Jensen, K.A.; Schneider, T. Sanding dust from nanoparticle-containing paints: Physical characterisation. J. Phys. Conf. Ser. 2009, 151, 012048. [Google Scholar] [CrossRef]
- Gomes, J.F.; Miranda, R.M.; Santos, T.J.; Carvalho, P.A. Emission of Nanoparticles During Friction Stir Welding (FSW) of Aluminium Alloys. J. Toxicol. Environ. Health Part A 2014, 77, 924–930. [Google Scholar] [CrossRef]
- Namgung, H.-G.; Kim, J.-B.; Woo, S.-H.; Park, S.; Kim, M.; Kim, M.-S.; Bae, G.-N.; Park, D.; Kwon, S.-B. Generation of Nanoparticles from Friction between Railway Brake Disks and Pads. Environ. Sci. Technol. 2016, 50, 3453–3461. [Google Scholar] [CrossRef] [PubMed]
- Schripp, T.; Wensing, M.; Uhde, E.; Salthammer, T.; He, C.; Morawska, L. Evaluation of Ultrafine Particle Emissions from Laser Printers Using Emission Test Chambers. Environ. Sci. Technol. 2008, 42, 4338–4343. [Google Scholar] [CrossRef] [PubMed]
- Göhler, D.; Stintz, M.; Hillemann, L.; Vorbau, M. Characterization of Nanoparticle Release from Surface Coatings by the Simulation of a Sanding Process. Ann. Occup. Hyg. 2010, 54, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Schlagenhauf, L.; Chu, B.T.T.; Buha, J.; Nüesch, F.; Wang, J. Release of Carbon Nanotubes from an Epoxy-Based Nanocomposite during an Abrasion Process. Environ. Sci. Technol. 2012, 46, 7366–7372. [Google Scholar] [CrossRef]
- Pang, C.; Neubauer, N.; Boyles, M.; Brown, D.; Kanase, N.; Hristozov, D.; Fernandes, T.; Stone, V.; Wohlleben, W.; Marcomini, A. Releases from transparent blue automobile coatings containing nanoscale copper phthalocyanine and their effects on J774 A1 macrophages. NanoImpact 2017, 7, 75–83. [Google Scholar] [CrossRef]
- Shandilya, N.; Le Bihan, O.; Bressot, C.; Morgeneyer, M. Emission of Titanium Dioxide Nanoparticles from Building Materials to the Environment by Wear and Weather. Environ. Sci. Technol. 2015, 49, 2163–2170. [Google Scholar] [CrossRef]
- Shandilya, N.; Le Bihan, O.; Bressot, C.; Morgeneyer, M. Evaluation of the Particle Aerosolization from n-TiO2 Photocatalytic Nanocoatings under Abrasion. J. Nanomater. 2014, 2014, 185080. [Google Scholar] [CrossRef] [Green Version]
- Sachse, S.; Silva, F.; Zhu, H.; Irfan, A.; Leszczyska, A.; Pielichowski, K.; Ermini, V.; Blazquez, M.; Kuzmenko, O.; Njuguna, J. The Effect of Nanoclay on Dust Generation during Drilling of PA6 Nanocomposites. J. Nanomater. 2012, 2012, 189386. [Google Scholar] [CrossRef]
- Park, I.; Kim, H.; Lee, S. Characteristics of tire wear particles generated in a laboratory simulation of tire/road contact conditions. J. Aerosol Sci. 2018, 124, 30–40. [Google Scholar] [CrossRef]
- Grigoratos, T.; Gustafsson, M.; Eriksson, O.; Martini, G. Experimental investigation of tread wear and particle emission from tyres with different treadwear marking. Atmos. Environ. 2018, 182, 200–212. [Google Scholar] [CrossRef]
- Zhao, B.; Wu, J. Modeling particle deposition from fully developed turbulent flow in ventilation duct. Atmos. Environ. 2006, 40, 457–466. [Google Scholar] [CrossRef]
- Koivisto, A.J.; Yu, M.; Hämeri, K.; Seipenbusch, M. Size resolved particle emission rates from an evolving indoor aerosol system. J. Aerosol. Sci. 2012, 47, 58–69. [Google Scholar] [CrossRef]
- Guha, A. A unified Eulerian theory of turbulent deposition to smooth and rough surfaces. J. Aerosol. Sci. 1997, 28, 1517–1537. [Google Scholar] [CrossRef]
- Zhao, B.; Wu, J. Modeling particle deposition onto rough walls in ventilation duct. Atmos. Environ. 2006, 40, 6918–6927. [Google Scholar] [CrossRef]
- Lai, A.C.K.; Nazaroff, W.W. Modeling Indoor Particle Deposition From Turbulent Flow Onto Smooth Surfaces. J. Aerosol. Sci. 2000, 31, 463–476. [Google Scholar] [CrossRef]
- Morency, F.; Hallé, S.; Dufresne, L.; Emond, C. Evaluation of diffusion models for airborne nanoparticles transport and dispersion. In Advances in Fluid Mechanics; WIT Press: Southampton, UK, 2008; Volume 59, pp. 111–120. [Google Scholar]
- Shi, S.; Zhao, B. Deposition of Indoor Airborne Particles onto Human Body Surfaces: A Modeling Analysis and Manikin-Based Experimental Study. Aerosol Sci. Technol. 2013, 47, 1363–1373. [Google Scholar] [CrossRef] [Green Version]
- Umezawa, M.; Sekita, K.; Suzuki, K.-i.; Kubo-Irie, M.; Niki, R.; Ihara, T.; Sugamata, M.; Takeda, K. Effect of aerosol particles generated by ultrasonic humidifiers on the lung in mouse. Part. Fibre Toxicol. 2013, 10, 64. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-s.; Friedlander, S.K.; Mädler, L. Nanoparticle aerosol science and technology: An overview. China Part. 2005, 3, 243–254. [Google Scholar] [CrossRef]
- Yamada, M.; Takaya, M.; Ogura, I. Performance evaluation of newly developed portable aerosol sizers used for nanomaterial aerosol measurements. Ind. Health 2015, 53, 511–516. [Google Scholar] [CrossRef] [Green Version]
- Appelquist, E.; Schlatter, P.; Alfredsson, P.H.; Lingwood, R.J. On the global nonlinear instability of the rotating-disk flow over a finite domain. J. Fluid Mech. 2016, 803, 332–355. [Google Scholar] [CrossRef] [Green Version]
- Childs, P.R.N. Chapter 6—Rotating Cylinders, Annuli, and Spheres. In Rotating Flow; Childs, P.R.N., Ed.; Butterworth-Heinemann: Oxford, UK, 2011; pp. 177–247. [Google Scholar] [CrossRef]
- Escudier, M.P. Observations of the flow produced in a cylindrical container by a rotating endwall. Exp. Fluids 1984, 2, 189–196. [Google Scholar] [CrossRef]
- Dixon, J.C. The Shock Absorber Handbook; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Johansen, S.T. The deposition of particles on vertical walls. Int. J. Multiph. Flow 1991, 17, 355–376. [Google Scholar] [CrossRef]
- Buonanno, G.; Dell’Isola, M.; Stabile, L.; Viola, A. Uncertainty Budget of the SMPS–APS System in the Measurement of PM1, PM2.5, and PM10. Aerosol Sci. Technol. 2009, 43, 1130–1141. [Google Scholar] [CrossRef] [Green Version]
- Wohlleben, W.; Meier, M.W.; Vogel, S.; Landsiedel, R.; Cox, G.; Hirth, S.; Tomović, Ž. Elastic CNT–polyurethane nanocomposite: Synthesis, performance and assessment of fragments released during use. Nanoscale 2013, 5, 369–380. [Google Scholar] [CrossRef] [Green Version]










| dp (nm) | τ1,j (s) | A1,j (cm3/s) | A2,j (cm3/s) | |
|---|---|---|---|---|
| 86.6 | 1137 ± 323 | 0.693 ± 0.197 | 2.30 ± 0.653 | 29.8 ± 6.90 |
| 115.5 | 1185 ± 160 | 0.158 ± 0.021 | 0.470 ± 0.0638 | 41.7 ± 8.80 |
| 154.0 | 1055 ± 107 | 1.72 ± 0.177 | 3.98 ± 0.410 | 50.6 ± 9.11 |
| 205.4 | 797 ± 205 | 6.32 ± 1.63 | 11.8 ± 3.04 | 60.4 ± 10.8 |
| 273.8 | 1123 ± 232 | 0.860 ± 0.178 | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nevshupa, R.; Castellote, M.; Cornelio, J.A.C.; Toro, A. Triboemission of FINE and Ultrafine Aerosol Particles: A New Approach for Measurement and Accurate Quantification. Lubricants 2020, 8, 21. https://doi.org/10.3390/lubricants8020021
Nevshupa R, Castellote M, Cornelio JAC, Toro A. Triboemission of FINE and Ultrafine Aerosol Particles: A New Approach for Measurement and Accurate Quantification. Lubricants. 2020; 8(2):21. https://doi.org/10.3390/lubricants8020021
Chicago/Turabian StyleNevshupa, Roman, Marta Castellote, Jesus Antonio Carlos Cornelio, and Alejandro Toro. 2020. "Triboemission of FINE and Ultrafine Aerosol Particles: A New Approach for Measurement and Accurate Quantification" Lubricants 8, no. 2: 21. https://doi.org/10.3390/lubricants8020021
APA StyleNevshupa, R., Castellote, M., Cornelio, J. A. C., & Toro, A. (2020). Triboemission of FINE and Ultrafine Aerosol Particles: A New Approach for Measurement and Accurate Quantification. Lubricants, 8(2), 21. https://doi.org/10.3390/lubricants8020021