Effects of Endophytic Entomopathogenic Ascomycetes on the Life-History Traits of Aphis gossypii Glover and Its Interactions with Melon Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Material: Aphid Colonies, Plants and Fungal Isolates
2.2. Inoculation of Melon Plants with Entomopathogenic Fungi and Verification of Endophytic Colonization
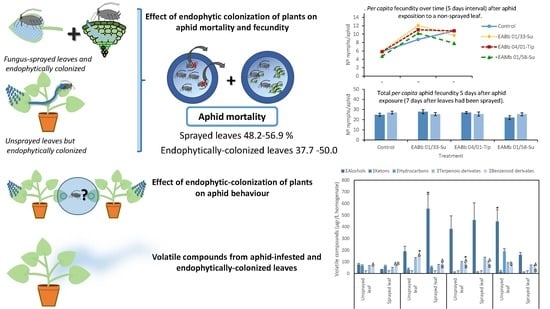
2.3. Effect of Endophytic Plant Colonization by Entomopathogenic Fungi on Aphid Mortality and Fecundity
2.4. Effect of Entomopathogenic Fungal Plant Colonization on Aphid Behavior
2.5. Analysis of Volatile Compounds from Aphid-Infested, Endophytically-Colonized Leaves
2.6. Statistical Analysis
3. Results
3.1. Endophytic Colonization of Melon Plants
3.2. Effect of Endophytic Colonization on Aphid Mortality
3.3. Effect of Endophytic Colonization of Plants on Aphid Fecundity
3.4. Effect of Endophytic-Colonization of Plants on Aphid Behavior
3.5. Volatile Compounds from Aphid-Infested and Endophytically-Colonized Leaves
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Van Emden, H.F.; Harrington, R. Aphids as Crop Pests, 2nd ed.; Cabi: Wallingford, UK, 2007. [Google Scholar]
- Dixon, A.F.G. Aphid Ecology: An Optimization Approach, 2nd ed.; Chapman & Hall: London, UK, 1998. [Google Scholar]
- Whalon, M.E.; Mota-Sánchez, D.; Hollingworth, R.M. The Arthropod Pesticide Resistance Database; Michigan State University: East Lansing, MI, USA, 2015. [Google Scholar]
- Brodeur, J.; Abram, P.; Heimpel, G.E.; Messing, R.H. Trends in biological control: Public interest, international networking and research direction. Biocontrol 2018, 63, 11–26. [Google Scholar] [CrossRef]
- Burges, H.D. Formulation of Mycoinsecticides. In Formulation of Microbial Biopesticides; Burges, H.D., Ed.; Kluwer Academic Publishes: Dordercht, The Netherlands, 1998; pp. 131–185. [Google Scholar]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132. [Google Scholar] [CrossRef] [PubMed]
- Quesada-Moraga, E.; Muñoz-Ledesma, F.J.; Santiago-Alvarez, C. Systemic protection of Papaver somniferum L. against Iraella luteipes (Hymenoptera: Cynipidae) by an endophytic strain of Beauveria bassiana (Ascomycota: Hypocreales). Environ. Entomol. 2009, 38, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, A.R.; Raya-Díaz, S.; Zamarreño, Á.M.; García-Mina, J.M.; Del Campillo, M.C.; Quesada-Moraga, E. An endophytic Beauveria bassiana strain increases spike production in bread and durum wheat plants and effectively controls cotton leafworm (Spodoptera littoralis) larvae. Biol. Control 2018, 116, 90–102. [Google Scholar] [CrossRef]
- Resquín-Romero, G.; Garrido-Jurado, I.; Delso, C.; Ríos-Moreno, A.; Quesada-Moraga, E. Transient endophytic colonization of plants improve the outcome of foliar applications of mycoinsecticides against chewing insects. J. Invertebr. Pathol. 2016, 136, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Jurado, I.; Resquín-Romero, G.; Amarilla, S.P.; Ríos-Moreno, A.; Carrasco, L.; Quesada-Moraga, E. Transient endophytic colonization of melon plants by entomopathogenic fungi after foliar application for the control of Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae). J. Pest Sci. 2017, 90, 319–330. [Google Scholar] [CrossRef]
- Vega, F.E. The use of fungal entomopathogens as endophytes in biological control: A review. Mycologia 2018, 110, 4–30. [Google Scholar] [CrossRef]
- Tjallingii, W.F. Electrical Recording of Stylet Penetration Activities. In Aphids: Their Biology. Natural Enemies and Control; Minks, A.K., Harrewijn, P.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1988; Volume 2B, pp. 95–108. [Google Scholar]
- Moreno, A.; Garzo, E.; Fernandez-Mata, G.; Kassem, M.; Aranda, M.A.; Fereres, A. Aphids secrete watery saliva into plant tissues from the onset of stylet penetration. Entomol. Exp. Appl. 2011, 139, 145–153. [Google Scholar] [CrossRef]
- Kim, J.J. Influence of Lecanicillium attenuatum on the development and reproduction of the cotton aphid, Aphis gossypii. Biocontrol 2007, 52, 789–799. [Google Scholar] [CrossRef]
- Gurulingappa, P.; McGee, P.A.; Sword, G. Endophytic Lecanicillium lecanii and Beauveria bassiana reduce the survival and fecundity of Aphis gossypii following contact with conidia and secondary metabolites. Crop Prot. 2011, 30, 349–353. [Google Scholar] [CrossRef]
- Akello, J.; Sikora, R. Systemic acropedal influence of endophyte seed treatment on Acyrthosiphon pisum and Aphis fabae offspring development and reproductive fitness. Biol. Control 2012, 61, 215–221. [Google Scholar] [CrossRef]
- Castillo-Lopez, D.; Zhu-Salzman, K.; Ek-Ramos, M.J.; Sword, G.A. The entomopathogenic fungal endophytes Purpureocillium lilacinum (formerly Paecilomyces lilacinus) and Beauveria bassiana negatively affect cotton aphid reproduction under both greenhouse and field conditions. PLoS ONE 2014, 9, e103891. [Google Scholar] [CrossRef] [PubMed]
- Clifton, E.H.; Jaronski, S.T.; Coates, B.S.; Hodgson, E.W.; Gassmann, A.J. Effects of endophytic entomopathogenic fungi on soybean aphid and identification of Metarhizium isolates from agricultural fields. PLoS ONE 2018, 13, e0194815. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Turlings, T.C.J. Plant volatiles as mate finding cues for insects. Trends Plant Sci. 2018, 23, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Yue, Q.; Wang, C.; Gianfagna, T.J.; Meyer, W.A. Volatile compounds of endophyte-free and infected tall fescue (Festuca arundinacea Schreb.). Phytochemistry 2001, 58, 935–941. [Google Scholar] [CrossRef]
- Hempel, S.; Stein, C.; Unsicker, S.B.; Renker, C.; Auge, H.; Weisser, W.W.; Buscot, F. Specific bottom-up effects of arbuscular mycorrhizal fungi across a plant-herbivore-parasitoid system. Oecologia 2009, 160, 267–277. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, M.; Erb, M.; Ton, J.; Brandenburg, A.; Karlen, D.; Zopfi, J.; Turlings, T.C.J. Volatiles produced by soil-borne endophytic bacteria increase plant pathogen resistance and affect tritrophic interactions. Plant Cell Environ. 2014, 37, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Shikano, I.; Rosa, C.; Tan, C.W.; Felton, G.W. Tritrophic interactions: Microbe-mediated plant effects on insect herbivores. Annu. Rev. Phytopathol. 2017, 55, 313–331. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; del-Val, E.; Macías-Rodríguez, L.; Alarcón, A.; González-Esquivel, C.E.; Larsen, J. Trichoderma atroviride, a maize root associated fungus, increases the parasitism rate of the fall armyworm Spodoptera frugiperda by its natural enemy Campoletis sonorensis. Soil Biol. Biochem. 2018, 122, 196–202. [Google Scholar] [CrossRef]
- Tasin, M.; Larsson-Herrera, S.; Knight, A.L.; Barros-Parada, W.; Fuentes-Contreras, E.; Pertot, I. Volatiles of grape inoculated with microorganisms: Modulation of grapevine moth oviposition and field attraction. Microb. Ecol. 2018, 76, 751–761. [Google Scholar] [CrossRef]
- Davis, T.S.; Crippen, T.L.; Hofstetter, R.W.; Tomberlin, J.K. Microbial volatile emissions as insect semiochemicals. J. Chem. Ecol. 2013, 39, 840–859. [Google Scholar] [CrossRef] [PubMed]
- Bojke, A.; Tkaczuk, C.; Stepnowski, P.; Golebiowski, M. Comparison of volatile compounds released by entomopathogenic fungi. Microbiol. Res. 2018, 214, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ortiz, A.; Pérez, A.G.; Sanz, C. Synthesis of aroma compounds of virgin olive oil: Significance of the cleavage of polyunsaturated fatty acid hydro- peroxides during the oil extraction process. Food Res. Int. 2013, 54, 1972–1978. [Google Scholar] [CrossRef]
- Kaplan, E.L.; Meier, P. Nonparametric estimation from incomplete observations. Am. Statist. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Stroup, W. Generalized Linear Mixed Models: Modern Concepts, Methods and Applications; CRC Press: London, UK, 2012. [Google Scholar]
- SAS/STAT® 9.3 User’s Guide; SAS Institute: Cary, NC, USA, 2011.
- IBM Corp. IBM SPSS Statistics for Windows, Version 24.0; IBM Corp: Armonk, NY, USA, 2016. [Google Scholar]
- Gurulingappa, P.; Sword, G.A.; Murdoch, G.; McGee, P.A. Colonization of crop plants by fungal entomopathogens and their effects on two insect pests when in planta. Biol. Control 2010, 55, 34–41. [Google Scholar] [CrossRef]
- Webb, T.J.; Hurd, H. Hymenolepis diminuta: Metacestode-induced reduction in the synthesis of the yolk protein, vitellogenin, in the fat body of Tenebrio Molit. Parasitol. 1996, 112, 429–436. [Google Scholar] [CrossRef]
- Kende, A.; Lim, P.P.; Lai, F.; Jessop, M.; Swindale, L.; Oliver, M.; Hurr, B.; Rickett, D.; Baxter, C. High throughput quantitative volatile profiling of melons with silicone rod extraction-thermal desorption-GC-MS for plant breeding line selection. Food Chem. 2019, 270, 368–374. [Google Scholar] [CrossRef] [PubMed]
- McCall, P.J.; Turlings, T.C.J.; Loughrin, J.H.; Proveaux, A.T.; Tumlinson, J.H. Herbivore-induced volatile emissions from cotton (Gossypium hirsutum L.) seedlings. J. Chem. Ecol. 1994, 20, 3039–3050. [Google Scholar] [CrossRef]
- Aartsma, Y.; Leroy, B.; van der Werf, W.; Dicke, M.; Poelman, E.H.; Bianchi, F.J.J.A. Intraspecific variation in herbivore-induced plant volatiles influences the spatial range of plant–parasitoid interactions. Oikos 2018, 128, 77–86. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Erb, M. Tritrophic interactions mediated by herbivore-induced plant volatiles: Mechanisms, ecological relevance, and application potential. Annu. Rev. Entomol. 2018, 63, 433–452. [Google Scholar] [CrossRef]
- Cai, X.M.; Sun, X.L.; Dong, W.X.; Wang, G.C.; Chen, Z.M. Herbivore species, infestation time, and herbivore density affect induced volatiles in tea plants. Chemoecology 2014, 24, 1. [Google Scholar] [CrossRef]
- Cruz-López, L.; Díaz-Díaz, B.; Rojas, J.C. Coffee volatiles induced after mechanical injury and beetle herbivory attract the coffee berry borer and two of its parasitoids. Arthropod Plant Interact. 2016, 10, 151. [Google Scholar] [CrossRef]
- Visser, J.H. Host odor perception in phytophagous insects. Annu. Rev. Entomol. 1986, 31, 121–144. [Google Scholar] [CrossRef]
- Cotes, B.; Rännbäck, L.M.; Björkman, M.; Norli, H.R.; Meyling, N.V.; Rämert, B.; Anderson, P. Habitat selection of a parasitoid mediated by volatiles informing on host and intraguild predator densities. Oecologia 2015, 79, 151–162. [Google Scholar] [CrossRef]
- Raguso, R.A.; Light, D.M. Electroantennogram responses of male Sphinx perelegans hawkmoths to floral and ‘green-leaf volatiles’. Entomol. Exp. Appl. 1998, 86, 287–293. [Google Scholar] [CrossRef]
- Hoballah, M.E.; Stuurman, J.; Turlings, T.C.J.; Guerin, P.M.; Connétable, S.; Kuhlemeier, C. The composition and timing of flower odour emission by wild Petunia axillaris coincide with the antennal perception and nocturnal activity of the pollinator. Manduca Sexta Planta 2005, 222, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; He, Y.; Xu, N.; Xia, Y.; Liu, Z. Isolation and identification of two strains of pathogenic bacteria and their effects on the volatile metabolites of Gracilariopsis lemaneiformis (Rhodophyta). J. Appl. Phycol. 2012, 24, 277–284. [Google Scholar] [CrossRef]
- Flath, R.A.; Cunningham, R.T.; Liquido, N.J.; McGovern, T.P. Alpha-ionol as attractant for trapping Bactrocera latifrons (Diptera:Tephritidae). J. Econ. Entomol. 1994, 87, 1470–1476. [Google Scholar] [CrossRef]
- Donaldson, J.M.I.; McGovern, T.P.; Ladd, J.R. Floral attractants for Cetoniinae and Rutelinae (Coleoptera: Scarabaeidae). J. Econ. Entomol. 1990, 83, 1298–1305. [Google Scholar] [CrossRef]
- Duan, S.G.; Li, D.Z.; Wang, M.Q. Chemosensory proteins used as target for screening behaviourally active compounds in the rice pest Cnaphalocrocis medinalis (Lepidoptera: Pyralidae). Insect Mol. Biol. 2019, 28, 123–135. [Google Scholar] [CrossRef]
- Obata, T.; Koh, H.S.; Kim, M.; Fukami, H. Constituents of plant hopper attractant in rice plant. Appl. Entomol. Zool. 1983, 18, 161–169. [Google Scholar] [CrossRef]
- Kepler, R.M.; Bruck, D.J. Examination of the interaction between the black vine weevil (Coleoptera: Curculionidae) and an entomopathogenic fungus reveals a new tritrophic interaction. Environ. Entomol. 2006, 35, 1021–1029. [Google Scholar] [CrossRef]
- Rondot, Y.; Reineke, A. Association of Beauveria bassiana with grapevine plants deters adult black vine weevils, Otiorhynchus sulcatus. Biocontr. Sci. Technol. 2017, 27, 811–820. [Google Scholar] [CrossRef]
- Sword, G.A.; Tessnow, A.; Ek-Ramos, M.J. Endophytic fungi alter sucking bug responses to cotton reproductive structures. Insect Sci. 2017, 24, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Rashki, M.; Shirvani, A. The effect of entomopathogenic fungus, Beauveria bassiana on life table parameters and behavioural response of Aphis gossypii. J. Insectol. 2013, 66, 85–91. [Google Scholar]






| Isolate | Fungal Species | Origin | Agroecosystem | Habitat | GenBank Accession Number | Spanish Type Culture Collection (CECT ) Accession Number |
|---|---|---|---|---|---|---|
| EABb 04/01-Tip | B. bassiana | Ecija (Sevilla, Spain) | Opium poppy crop | Insect (Iraella luteipes) | FJ972963 | 20744 |
| EABb 01/33-Su | B. bassiana | El Bosque (Cádiz, Spain) | Traditional olive Orchard | Soil | FJ972969 | - |
| EAMa 01/58–Su | M. brunneum | Hinojosa del Duque (Córdoba, Spain) | Wheat crop | Soil | JN900390 | 20764 |
| Treatment | Mortality (%) | Kaplan-Meier Survival Analysis | ||
|---|---|---|---|---|
| Mean (± SE) a | AST b (± SE; Days) | Confidence Interval (95%) | ||
| Lower Limit | Upper Limit | |||
| Control | 0.80 ± 0.06 | 6.56 ± 0.25 a | 6.07 | 7.05 |
| EABb 01/33-Su | 48.15 ± 0.18 * | 6.15 ± 0.28 b | 5.60 | 6.71 |
| EABb 04/01-Tip | 56.92 ± 0.18 * | 6.18 ± 0.25 b | 5.70 | 6.66 |
| EAMa 01/58-Su | 53.68 ± 0.18 * | 5.77 ± 0.28 b | 5.22 | 6.33 |
| Treatment | Mortality (%) | Kaplan-Meier Survival Analysis | ||
|---|---|---|---|---|
| Mean (± SE) a | AST b (± SE; Days) | Confidence Interval (95%) | ||
| Lower Limit | Upper Limit | |||
| Control | 13.74 ± 0.08 | 6.62 ± 0.21 a | 6.22 | 7.03 |
| EABb 01/33-Su | 49.99 ± 14.09 * | 5.90± 0.32 b | 5.28 | 6.52 |
| EABb 04/01-Tip | 37.71 ± 13.61 | 6.41 ± 0.28 a | 5.85 | 6.96 |
| EAMa 01/58-Su | 40.08 ± 13.56 | 6.11 ± 0.25 b | 5.62 | 6.60 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Mas, N.; Sánchez-Ortiz, A.; Valverde-García, P.; Quesada-Moraga, E. Effects of Endophytic Entomopathogenic Ascomycetes on the Life-History Traits of Aphis gossypii Glover and Its Interactions with Melon Plants. Insects 2019, 10, 165. https://doi.org/10.3390/insects10060165
González-Mas N, Sánchez-Ortiz A, Valverde-García P, Quesada-Moraga E. Effects of Endophytic Entomopathogenic Ascomycetes on the Life-History Traits of Aphis gossypii Glover and Its Interactions with Melon Plants. Insects. 2019; 10(6):165. https://doi.org/10.3390/insects10060165
Chicago/Turabian StyleGonzález-Mas, Natalia, Araceli Sánchez-Ortiz, Pablo Valverde-García, and Enrique Quesada-Moraga. 2019. "Effects of Endophytic Entomopathogenic Ascomycetes on the Life-History Traits of Aphis gossypii Glover and Its Interactions with Melon Plants" Insects 10, no. 6: 165. https://doi.org/10.3390/insects10060165
APA StyleGonzález-Mas, N., Sánchez-Ortiz, A., Valverde-García, P., & Quesada-Moraga, E. (2019). Effects of Endophytic Entomopathogenic Ascomycetes on the Life-History Traits of Aphis gossypii Glover and Its Interactions with Melon Plants. Insects, 10(6), 165. https://doi.org/10.3390/insects10060165