How Bees Respond Differently to Field Margins of Shrubby and Herbaceous Plants in Intensive Agricultural Crops of the Mediterranean Area
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Setting of the Experiment
2.2. Sampling of Bees and Vegetation
2.3. Analysis of Data
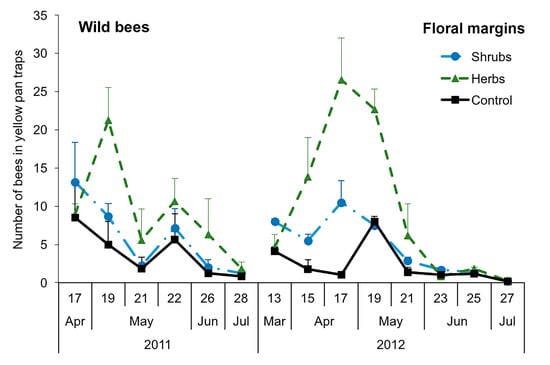
3. Results
3.1. Diversity of Bees
3.2. Abundance and Dynamics of Bees in Margins: Pan Traps and Visual Samplings
3.3. Structure of Bee Communities and Floral Resources
4. Discussion
4.1. Floral Margins and Foraging Behaviour of Bees
4.2. Bee Diversity and Conservation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, R.A.; Sutherland, W.J. Post-war changes in arable farming and biodiversity in Great Britain. J. Appl. Ecol. 2002, 39, 157–176. [Google Scholar] [CrossRef] [Green Version]
- Petanidou, T.; Kizos, T.; Soulakellis, N. Socioeconomic dimensions of changes in the agricultural landscape of the Mediterranean basin: A case study of the abandonment of cultivation terraces on Nisyros Island, Greece. Environ. Manag. 2008, 41, 250–266. [Google Scholar] [CrossRef] [PubMed]
- Kremen, C.; Williams, N.M.; Thorp, R.W. Crop pollination from native bees at risk from agricultural intensification. Proc. Natl. Acad. Sci. USA 2002, 99, 16812–16816. [Google Scholar] [CrossRef] [Green Version]
- Ghazoul, J. Buzziness as usual? Questioning the global pollination crisis. Trends Ecol. Evol. 2005, 20, 367–373. [Google Scholar] [CrossRef]
- Steffan-Dewenter, I.; Potts, S.G.; Packer, L. Pollinator diversity and crop pollination services are at risk. Trends Ecol. Evol. 2005, 20, 651–653. [Google Scholar] [CrossRef]
- Biesmeijer, J.C.; Roberts, S.P.M.; Reemer, M.; Ohlemuller, R.; Edwards, M.; Peeters, T.; Schaffers, A.P.; Potts, S.G.; Kleukers, R.; Thomas, C.D.; et al. Parallel Declines in Pollinators and Insect-Pollinated Plants in Britain and the Netherlands. Science 2006, 313, 351–354. [Google Scholar] [CrossRef]
- Williams, P.H.; Osborne, J.L. Bumblebee vulnerability and conservation world-wide. Apidologie 2009, 40, 367–387. [Google Scholar] [CrossRef] [Green Version]
- Le Féon, V.; Schermann-Legionnet, A.; Delettre, Y.; Aviron, S.; Billeter, R.; Bugter, R.; Hendrickx, F.; Burel, F. Intensification of agriculture, landscape composition and wild bee communities: A large scale study in four European countries. Agric. Ecosyst. Environ. 2010, 137, 143–150. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Koh, I.; Lonsdorf, E.V.; Williams, N.M.; Brittain, C.; Isaacs, R.; Gibbs, J.; Ricketts, T.H. Modeling the status, trends, and impacts of wild bee abundance in the United States. Proc. Natl. Acad. Sci. USA 2016, 113, 140–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera, C.M. Variation in mutualisms: The spatio—temporal mosaic of a pollinator assemblage. Biol. J. Linn. Soc. 1988, 35, 95–125. [Google Scholar] [CrossRef]
- Petanidou, T.; Ellis, W.N. Pollinating fauna of a phryganic ecosystem: Composition and diversity. Biodivers. Lett. 1993, 1, 9–22. [Google Scholar] [CrossRef]
- Pérez-Marcos, M.; Ortiz-Sánchez, F.J.; López-Gallego, E.; Ramírez-Soria; Sanchez, J.A. The importance of the qualitative composition of floral margins to the maintenance of rich communities of bees. IOBC/WPRS Bull. 2017, 122, 83–87. [Google Scholar]
- Richards, A.J. Does Low Biodiversity Resulting from Modern Agricultural Practice Affect Crop Pollination and Yield? Ann. Bot. 2001, 88, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Steffan-Dewenter, I.; Münzenberg, U.; Bürger, C.; Thies, C.; Tscharntke, T. Scale-dependent effects of landscape context on three pollinator guilds. Ecology 2002, 83, 1421–1432. [Google Scholar] [CrossRef]
- Steffan-Dewenter, I.; Klein, A.M.; Alfert, T.; Gaebele, V.; Tscharntke, T. Bee diversity and plant–pollinator interactions in fragmented landscapes. In Specialization and Generalization in Plant–Pollinator Interactions; M.Waser, N., Ollerton, J., Eds.; Chicago Press: Chicago, IL, USA, 2006; pp. 387–408. [Google Scholar]
- Hendrickx, F.; Maelfait, J.P.; Van Wingerden, W.; Schweiger, O.; Speelmans, M.; Aviron, S.; Augenstein, I.; Billeter, R.; Bailey, D.; Bukacek, R.; et al. How landscape structure, land-use intensity and habitat diversity affect components of total arthropod diversity in agricultural landscapes. J. Appl. Ecol. 2007, 44, 340–351. [Google Scholar] [CrossRef]
- Ricketts, T.H.; Regetz, J.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Bogdanski, A.; Gemmill-Herren, B.; Greenleaf, S.S.; Klein, A.M.; Mayfield, M.M.; et al. Landscape effects on crop pollination services: Are there general patterns? Ecol. Lett. 2008, 11, 499–515. [Google Scholar] [CrossRef]
- Brown, M.J.F.; Paxton, R.J. The conservation of bees: A global perspective. Apidologie 2009, 40, 410–416. [Google Scholar] [CrossRef] [Green Version]
- Winfree, R.; Aguilar, R.; Vázquez, D.P.; LeBuhn, G.; Aizen, M.A. A meta-analysis of bees’ responses to anthropogenic disturbance. Ecology 2009, 90, 2068–2076. [Google Scholar] [CrossRef]
- Montero-Castaño, A.; Vilà, M. Impact of landscape alteration and invasions on pollinators: A meta-analysis. J. Ecol. 2012, 100, 884–893. [Google Scholar] [CrossRef]
- McKechnie, I.M.; Thomsen, C.J.M.; Sargent, R.D. Forested field edges support a greater diversity of wild pollinators in lowbush blueberry (Vaccinium angustifolium). Agric. Ecosyst. Environ. 2017, 237, 154–161. [Google Scholar] [CrossRef]
- Ortiz-Sánchez, F.J.; Belda, J. Fenología de una comunidad de Apoidea (Hymenoptera) en medio agrícola en el sreste de España. Boletín De Sanid. Veg. Plagas 1994, 20, 725–735. [Google Scholar]
- Klein, A.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. Biol. Sci. R. Soc. 2007, 274, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Memmott, J.; Craze, P.G.; Waser, N.M.; Price, M. V Global warming and the disruption of plant–pollinator interactions. Ecol. Lett. 2007, 10, 710–717. [Google Scholar] [CrossRef]
- Goulson, D.; Lye, G.C.; Darvill, B. Decline and Conservation of Bumble Bees. Annu. Rev. Entomol. 2008, 53, 191–208. [Google Scholar] [CrossRef]
- Földesi, R.; Kovács-Hostyánszki, A.; Korösi, Á.; Somay, L.; Elek, Z.; Markó, V.; Sárospataki, M.; Bakos, R.; Varga, Á.; Nyisztor, K.; et al. Relationships between wild bees, hoverflies and pollination success in apple orchards with different landscape contexts. Agric. For. Entomol. 2016, 18, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Morrison, J.; Izquierdo, J.; Plaza, E.H.; González-Andújar, J.L.; Hernández, E.; González-Andújar, J.L. The role of field margins in supporting wild bees in Mediterranean cereal agroecosystems: Which biotic and abiotic factors are important? Agric. Ecosyst. Environ. 2017, 247, 216–224. [Google Scholar] [CrossRef]
- Warzecha, D.; Diekötter, T.; Wolters, V.; Jauker, F. Attractiveness of wildflower mixtures for wild bees and hoverflies depends on some key plant species. Insect Conserv. Divers. 2018, 11, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Gabriel, D.; Tscharntke, T. Insect pollinated plants benefit from organic farming. Agric. Ecosyst. Environ. 2007, 118, 43–48. [Google Scholar] [CrossRef]
- Holzschuh, A.; Steffan-Dewenter, I.; Tscharntke, T. Agricultural landscapes with organic crops support higher pollinator diversity. Oikos 2008, 117, 354–361. [Google Scholar] [CrossRef]
- Gill, R.J.; Raine, N.E. Chronic impairment of bumblebee natural foraging behaviour induced by sublethal pesticide exposure. Funct. Ecol. 2014, 28, 1459–1471. [Google Scholar] [CrossRef]
- Sydenham, M.A.K.; Eldegard, K.; Totland, Ø. Spatio-temporal variation in species assemblages in field edges: Seasonally distinct responses of solitary bees to local habitat characteristics and landscape conditions. Biodivers. Conserv. 2014, 23, 2393–2414. [Google Scholar] [CrossRef]
- Morandin, L.A.; Winston, M.L.; Franklin, M.T.; Abbott, V.A. Lethal and sub-lethal effects of spinosad on bumble bees (Bombus impatiens Cresson). Pest Manag. Sci. 2005, 61, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Alston, D.G.; Tepedino, V.J.; Bradley, B.A.; Toler, T.R.; Griswold, T.L.; Messinger, S.M. Effects of the insecticide phosmet on solitary bee foraging and nesting in orchards of Capitol Reef National Park, Utah. Environ. Entomol. 2007, 36, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Brittain, C.A.; Vighi, M.; Bommarco, R.; Settele, J.; Potts, S.G. Impacts of a pesticide on pollinator species richness at different spatial scales. Basic Appl. Ecol. 2010, 11, 106–115. [Google Scholar] [CrossRef]
- Sgolastra, F.; Medrzycki, P.; Bortolotti, L.; Renzi, T.; Tosi, S.; Bogo, G.; Teper, D.; Porrini, C.; Bosch, J.; Renzi, M.T.; et al. Synergistic mortality between a neonicotinoid insecticide and an ergosterol-biosynthesis-inhibiting fungicide in three bee species. Pest Manag. Sci. 2017, 73, 1236–1243. [Google Scholar] [CrossRef]
- Sgolastra, F.; Blasioli, S.; Renzi, T.; Tosi, S.; Medrzycki, P.; Molowny-Horas, R.; Porrini, C.; Braschi, I. Chemosphere Lethal effects of Cr(III) alone and in combination with propiconazole and clothianidin in honey bees. Chemosphere 2018, 191, 365–372. [Google Scholar] [CrossRef]
- Losey, J.E.; Vaughan, M. The economic value of ecological services provided by insects. Bioscience 2006, 56, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Kearns, C.A.; Inouye, D.W.; Waser, N.M. Endangered Mutualism: The Conservation of Plant-Pollinator Interactions. Annu. Rev. Ecol. Syst. 1998, 29, 83–112. [Google Scholar] [CrossRef]
- Michener, C.D. The Bees of the World, 2nd ed.; The Johns Hopkins University Press: Baltimore, MD, USA, 2007; ISBN 0-8018-8573-6. [Google Scholar]
- Westphal, C.; Bommarco, R.; Carré, G.; Lamborn, E.; Morison, N.; Petanidou, T.; Potts, S.G.; Roberts, S.P.M.; Szentgyörgyi, H.; Tscheulin, T.; et al. Measuring bee diversity in different European habitats and biogeographical regions. Ecol. Monogr. 2008, 78, 653–671. [Google Scholar] [CrossRef] [Green Version]
- Neumann, P.; Carreck, N.L. Honey bee colony losses. J. Apic. Res. 2010, 49, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Cameron, S.A.; Lozier, J.D.; Strange, J.P.; Koch, J.B.; Cordes, N.; Solter, L.F.; Griswold, T.L. Patterns of widespread decline in North American bumble bees. Proc. Natl. Acad. Sci. USA 2011, 108, 662–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, A.; Steffan-Dewenter, I.; Westphal, C.; Messinger, O.; Potts, S.G.; Roberts, S.P.M.; Settele, J.; Szentgyörgyi, H.; Vaissière, B.E.; Vaitis, M.; et al. Assessing bee species richness in two Mediterranean communities: Importance of habitat type and sampling techniques. Ecol. Res. 2011, 26, 969–983. [Google Scholar] [CrossRef]
- Carvalheiro, L.G.; Kunin, W.E.; Keil, P.; Aguirre-Gutierrez, J.; Ellis, W.N.; Fox, R.; Groom, Q.; Hennekens, S.; Van Landuyt, W.; Maes, D.; et al. Species richness declines and biotic homogenisation have slowed down for NW-European pollinators and plants. Ecol. Lett. 2013, 16, 870–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieto, A.; Roberts, S.P.M.; Kemp, J.; Rasmont, P.; Kuhlmann, M.; García Criado, M.; Biesmeijer, J.C.; Bogusch, P.; Dathe, H.H.; De la Rúa, P.; et al. European Red List of Bees; Publication Office of the European Union: Luxembourg, 2014; ISBN 9789279445125. [Google Scholar]
- Campbell, P.J. Declining European bee health: Banning the neonicotinoids is not the answer. Outlooks Pest Manag. 2013, 24, 52–57. [Google Scholar] [CrossRef]
- Letourneau, D.K. Conservation Biology: Lessons for conserving natural enemies. In Conservation Biological Control; Barbosa, P., Ed.; Academic Press: New York, NY, USA, 1998; pp. 9–38. ISBN 9780120781478. [Google Scholar]
- Gurr, G.M.; Wratten, S.D.; Michael Luna, J. Multi-function agricultural biodiversity: Pest management and other benefits. Basic Appl. Ecol. 2003, 4, 107–116. [Google Scholar] [CrossRef]
- Tscharntke, T.; Klein, A.M.; Kruess, A.; Steffan-Dewenter, I.; Thies, C. Landscape perspectives on agricultural intensification and biodiversity—ecosystem service management. Ecol. Lett. 2005, 8, 857–874. [Google Scholar] [CrossRef]
- Bianchi, F.J.J.A.; Booij, C.J.H.; Tscharntke, T. Sustainable pest regulation in agricultural landscapes: A review on landscape composition, biodiversity and natural pest control. Proc. R. Soc. B-Biol. Sci. 2006, 273, 1715–1727. [Google Scholar] [CrossRef] [Green Version]
- Rey Benayas, J.M.; Newton, A.C.; Diaz, A.; Bullock, J.M. Enhancement of biodiversity and ecosystem services by ecological restoration: A meta-analysis. Science 2009, 325, 1121–1124. [Google Scholar] [CrossRef]
- Wratten, S.D.; Gillespie, M.; Decourtye, A.; Mader, E.; Desneux, N. Pollinator habitat enhancement: Benefits to other ecosystem services. Agric. Ecosyst. Environ. 2012, 159, 112–122. [Google Scholar] [CrossRef]
- Carreck, N.L.; Williams, I.H. Food for insect pollinators on farmland: Insect visits to flowers of annual seed mixtures. J. Insect Conserv. 2002, 6, 13–23. [Google Scholar] [CrossRef]
- Pywell, R.F.; Warman, E.A.; Carvell, C.; Sparks, T.H.; Dicks, L.V.; Bennett, D.; Wright, A.; Critchley, C.N.R.; Sherwood, A. Providing foraging resources for bumblebees in intensively farmed landscapes. Biol. Conserv. 2005, 121, 479–494. [Google Scholar] [CrossRef]
- Russo, L.; Debarros, N.; Yang, S.; Shea, K.; Mortensen, D. Supporting crop pollinators with floral resources: Network-based phenological matching. Ecol. Evol. 2013, 3, 3125–3140. [Google Scholar] [CrossRef]
- Campbell, A.J.; Wilby, A.; Sutton, P.; Wackers, F.L. Do sown flower strips boost wild pollinator abundance and pollination services in a spring- flowering crop? A case study from UK cider apple orchards. Agric. Ecosyst. Environ. 2017, 239, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Pisanty, G.; Mandelik, Y. Profiling crop pollinators: Life-history traits predict habitat use and crop visitation by Meditteranean wild bees. Ecol. Appl. 2015, 25, 742–752. [Google Scholar] [CrossRef]
- Ghazoul, J. Floral diversity and the facilitation of pollination. J. Ecol. 2006, 94, 295–304. [Google Scholar] [CrossRef]
- Greenleaf, S.S.; Kremen, C. Wild bees enhance honey bees’ pollination of hybrid sunflower. Proc. Natl. Acad. Sci. USA 2006, 103, 13890–13895. [Google Scholar] [CrossRef] [Green Version]
- Winfree, R.; Williams, N.M.; Gaines, H.; Ascher, J.S.; Kremen, C. Wild bee pollinators provide the majority of crop visitation across land-use gradients in New Jersey and Pennsylvania, USA. J. Appl. Ecol. 2008, 45, 793–802. [Google Scholar] [CrossRef]
- Ebeling, A.; Klein, A.M.; Schumacher, J.; Weisser, W.W.; Tscharntke, T. How does plant richness affect pollinator richness and temporal stability of flower visits? Oikos 2008, 117, 1808–1815. [Google Scholar] [CrossRef]
- Sanchez, J.A.; Carrasco, A.; La-Spina, M.; Ibáñez, H.; Canomanuel, G.; Ortiz-Sánchez, F.J.; López, E.; Lacasa, A. Edges of natural vegetation to increase the diversity of wild bees in agricultural field margins. IOBC-WPRS Bull. 2014, 100, 117–121. [Google Scholar]
- M’Gonigle, L.K.; Ponisio, L.C.; Cutler, K.; Kremen, C. Habitat restoration promotes pollinator persistence and colonization in intensively managed agriculture. Ecol. Appl. 2015, 25, 1557–1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, T.J.; Holland, J.M.; Hughes, W.O.H.; Goulson, D. Targeted agri-environment schemes significantly improve the population size of common farmland bumblebee species. Mol. Ecol. 2015, 24, 1668–1680. [Google Scholar] [CrossRef] [PubMed]
- Kleijn, D.; Winfree, R.; Bartomeus, I.; Carvalheiro, L.G.; Henry, M.; Isaacs, R.; Klein, A.-M.M.; Kremen, C.; M’Gonigle, L.K.; Rader, R.; et al. Delivery of crop pollination services is an insufficient argument for wild pollinator conservation. Nat. Commun. 2015, 6, 7414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prys-Jones, O.E.; Corbet, S.A. Bumblebees, 2nd ed.; Richmond Publishing Co.: Slough, UK, 1991; ISBN 0855462574. [Google Scholar]
- Lagerlöf, J.; Stark, J.; Svensson, B. Margins of agricultural fields as habitats for pollinating insects. Agric. Ecosyst. Environ. 1992, 40, 117–124. [Google Scholar] [CrossRef]
- Lagerlöf, J.; Wallin, H. The abundance of arthropods along two field margins with different types of vegetation composition: An experimental study. Agric. Ecosyst. Environ. 1993, 43, 141–154. [Google Scholar] [CrossRef]
- Carreck, N.L.; Williams, I.H. Observations on two commercial flower mixtures as food sources for beneficial insects in the UK. J. Agric. Sci. 1997, 128, 397–403. [Google Scholar] [CrossRef]
- Cheesman, O.D. The impact of some field boundary management practices on the development of Dipsacus fullonum L. flowering stems, and implications for conservation. Agric. Ecosyst. Environ. 1998, 68, 41–49. [Google Scholar] [CrossRef]
- Bäckman, J.C.; Tiainen, J. Habitat quality of field margins in a Finnish farmland area for bumblebees (Hymenoptera: Bombus and Psithyrus). Agric. Ecosyst. Environ. 2002, 89, 53–68. [Google Scholar] [CrossRef]
- Carvell, C.; Meek, W.R.; Pywell, R.F.; Nowakowski, M. The response of foraging bumblebees to successional change in newly created arable field margins. Biol. Conserv. 2004, 118, 327–339. [Google Scholar] [CrossRef]
- Croxton, P.J.; Hann, J.P.; Greatorex-Davies, J.N.; Sparks, T.H. Linear hotspots? The floral and butterfly diversity of green lanes. Biol. Conserv. 2005, 121, 579–584. [Google Scholar] [CrossRef]
- Hannon, L.E.; Sisk, T.D. Hedgerows in an agri-natural landscape: Potential habitat value for native bees. Biol. Conserv. 2009, 142, 2140–2154. [Google Scholar] [CrossRef]
- Morandin, L.A.; Kremen, C. Hedgerow restoration promotes pollinator populations and exports native bees to adjacent fields. Ecol. Appl. 2013, 23, 829–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potts, S.G.; Vulliamy, B.; Dafni, A.; NE’Eman, G.; Willmer, P. Linking bees and flowers; How do floral communities stucture pollinator comunities? Ecology 2003, 84, 2628–2642. [Google Scholar] [CrossRef] [Green Version]
- Carvell, C.; Westrich, P.; Meek, W.R.; Pywell, R.F.; Nowakowski, M. Assessing the value of annual and perennial forage mixtures for bumblebees by direct observation and pollen analysis. Apidologie 2006, 37, 326–340. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.D.; Steiner, K.E. Generalization versus specialization in plant pollination systems. Trends Ecol. Evol. 2000, 15, 140–143. [Google Scholar] [CrossRef]
- Fenster, C.B.; Armbruster, W.S.; Wilson, P.; Dudash, M.R.; Thomson, J.D. Pollination Syndromes and Floral Specialization. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 375–403. [Google Scholar] [CrossRef]
- Petanidou, T.; Lamborn, E. A land for flowers and bees: Studying pollination ecology in Mediterranean communities. Plant Biosyst. 2005, 139, 279–294. [Google Scholar] [CrossRef]
- Potts, S.G.; Petanidou, T.; Roberts, S.; O’Toole, C.; Hulbert, A.; Willmer, P. Plant-pollinator biodiversity and pollination services in a complex Mediterranean landscape. Biol. Conserv. 2006, 129, 519–529. [Google Scholar] [CrossRef]
- Petanidou, T.; Ståhls, G.; Vujić, A.; Olesen, J.M.; Rojo, S.; Thrasyvoulou, A.; Sgardelis, S.; Kallimanis, A.S.; Kokkini, S.; Tscheulin, T. Investigating plant—pollinator relationships in the Aegean: The approaches of the project POL-AEGIS (The pollinators of the Aegean archipelago: Diversity and threats). J. Apic. Res. 2013, 52, 106–117. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Sánchez, F.J.; Aguirre-Segura, A. Efecto del color sobre las capturas de abejas mediante trampas Moericke en el sur de España (Hymeoptera, Apoidea). Graelsia 1993, 49, 63–71. [Google Scholar]
- R-Development-Core-Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Hothorn, T.; Bret, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-6. 2019. Available online: https://CRAN.R-project (accessed on 21 April 2017).
- Nicholls, C.I.; Altieri, M.A. Plant biodiversity enhances bees and other insect pollinators in agroecosystems. A review. Agron. Sustain. Dev. 2012, 33, 257–274. [Google Scholar] [CrossRef] [Green Version]
- Winfree, R. The conservation and restoration of wild bees. Ann. N.Y. Acad. Sci. 2010, 1195, 169–197. [Google Scholar] [CrossRef]
- Batáry, P.; Báldi, A.; Kleijn, D.; Tscharntke, T. Landscape-moderated biodiversity effects of agri-environmental management: A meta-analysis. Proc. R. Soc. B Biol. Sci. 2011, 278, 1894–1902. [Google Scholar] [CrossRef]
- Kennedy, C.M.; Lonsdorf, E.; Neel, M.C.; Williams, N.M.; Ricketts, T.H.; Winfree, R.; Bommarco, R.; Brittain, C.; Burley, A.L.; Cariveau, D.; et al. A global quantitative synthesis of local and landscape effects on wild bee pollinators in agro ecosystems. Ecol. Lett. 2013, 16, 584–599. [Google Scholar] [CrossRef]
- Blaauw, B.R.; Isaacs, R. Larger patches of diverse floral resources increase insect pollinator density, diversity, and their pollination of native wildflowers. Basic Appl. Ecol. 2014, 15, 701–711. [Google Scholar] [CrossRef]
- Harmon-Threatt, A.N.; Hendrix, S.D. Prairie restorations and bees: The potential ability of seed mixes to foster native bee communities. Basic Appl. Ecol. 2015, 16, 64–72. [Google Scholar] [CrossRef]
- Carvell, C.; Meek, W.R.; Pywell, R.F.; Goulson, D.; Nowakowski, M. Comparing the efficacy of agri-environment schemes to enhance bumble bee abundance and diversity on arable field margins. J. Appl. Ecol. 2007, 44, 29–40. [Google Scholar] [CrossRef]
- Carvell, C.; Osborne, J.L.; Bourke, A.F.G.; Freeman, S.N.; Pywell, R.F.; Heard, M.S. Bumble bee species’ responses to a targeted conservation measure depend on landscape context and habitat quality. Ecol. Appl. 2011, 21, 1760–1771. [Google Scholar] [CrossRef] [Green Version]
- Heard, M.S.; Carvell, C.; Carreck, N.L.; Rothery, P.; Osborne, J.L.; Bourke, A.F.G. Landscape context not patch size determines bumble-bee density on flower mixtures sown for agri-environment schemes. Biol. Lett. 2007, 3, 638–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohler, F.; Verhulst, J.; Van Klink, R.; Kleijn, D. At what spatial scale do high-quality habitats enhance the diversity of forbs and pollinators in intensively farmed landscapes? J. Appl. Ecol. 2008, 45, 753–762. [Google Scholar] [CrossRef]
- Hopwood, J.L. The contribution of roadside grassland restorations to native bee conservation. Biol. Conserv. 2008, 141, 2632–2640. [Google Scholar] [CrossRef]
- Scheper, J.; Holzschuh, A.; Kuussaari, M.; Potts, S.G.; Rundlöf, M.; Smith, H.G.; Kleijn, D. Environmental factors driving the effectiveness of European agri-environmental measures in mitigating pollinator loss - a meta-analysis. Ecol. Lett. 2013, 16, 912–920. [Google Scholar] [CrossRef]
- Mayer, C. Does Grazing Influence Bee Diversity? In African Biodiversity: Molecules, Organisms, Ecosystems; Huber, B.A., Sinclair, B.J., Lampe, K.-H., Eds.; Springer: Berlin, Germany, 2005; pp. 173–179. [Google Scholar]
- Wilson, J.S.; Griswold, T.; Messinger, O.J. Sampling Bee Communities (Hymenoptera: Apiformes) in a Desert Landscape: Are Pan Traps Sufficient? J. Kans. Entomol. Soc. 2008, 81, 288–300. [Google Scholar] [CrossRef]
- Petanidou, T.; Ellis, W.N. Interdependence of native bee faunas and floras in changing Mediterranean communities. In The Conservation of Bees; Matheson, A., Buchmann, S.L., O’Toole, C.P.W., Williams, I.H., Eds.; Academic Press: London, UK, 1996; pp. 201–226. [Google Scholar]
- Ortiz-Sánchez, F.J.; Aguirre-Segura, A. Estructura y dinámica estacional de una comunidad de Apoidea (Hymenoptera) en Almería. Eos 1991, 67, 3–22. [Google Scholar]
- Ortiz-Sánchez, F.J.; Aguirre-Segura, A. Comparación de la eficacia de diferentes alturas en la captura de abejas mediante el empleo de trampas de Moericke (Hymenoptera: Apoidea). Graelsia 1992, 48, 35–43. [Google Scholar]
- Ortiz-Sánchez, F.J. Lista actualizada de las especies de abejas de España (Hymenoptera: Apoidea: Apiformes). Boletín De La Soc. Entomológica Aragonesa 2011, 49, 265–281. [Google Scholar]
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global consequences of land use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef] [Green Version]
- Kremen, C.; Ricketts, T. Global Perspectives on Pollination Disruptions. Conserv. Biol. 2000, 14, 1226–1228. [Google Scholar] [CrossRef]
- Patiny, S.; Rasmont, P.; Michez, D. A survey and review of the status of wild bees in the West-Palaearctic region. Apidologie 2009, 40, 313–331. [Google Scholar] [CrossRef] [Green Version]






| Family | Herbaceous Plants | g/m2 | Family | Shrubby Plants | N/m2 |
|---|---|---|---|---|---|
| Boraginaceae | Borago officinalis | 0.50 | Fabaceae | Anthyllis cytisoides | 0.10 |
| Asteraceae | Calendula officinalis | 1.13 | Lamiaeae | Ballota hirsuta | 0.10 |
| Brassicaceae | Diplotaxis catholica | 0.10 | Fabaceae | Dorycnium pentaphyllum | 0.10 |
| Apiaceae | Daucus sp. | 1.00 | Fabaceae | Genista umbellata | 0.15 |
| Boraginaceae | Echium vulgare | 0.25 | Lamiaceae | Lavandula dentata | 0.25 |
| Fabaceae | Medicago sativa | 0.63 | Lamiaceae | Lavandula stoechas | 0.25 |
| Fabaceae | Melilotus officinalis | 0.63 | Lamiaceae | Phlomis purpurea | 0.25 |
| Ranunculaceae | Nigella damascena | 0.25 | Lamiaceae | Rosmarinus officinalis | 0.10 |
| Lamiaceae | Salvia verbenaca | 0.50 | Lamiaceae | Salvia officinalis | 0.10 |
| Caryophyllaceae | Silene vulgaris | 0.50 | Asteraceae | Santolina chamaecyparisus | 0.10 |
| Fabaceae | Vicia sativa | 0.50 | Lamiaceae | Thymus vulgaris | 0.25 |
| Family | Species | St | Margin | Tot | % | ||
|---|---|---|---|---|---|---|---|
| S | H | C | |||||
| Andrenidae | Andrena asperrima Pérez, 1895 | LC | 2 | 1 | 0 | 3 | 0.2 |
| Andrena ferrugineicrus Dours, 1872 | LC | 0 | 10 | 0 | 10 | 0.6 | |
| Andrena flavipes Panzer, 1799 | LC | 17 | 62 | 10 | 89 | 5.7 | |
| Andrena humilis Imhoff, 1832 | DD | 7 | 16 | 1 | 24 | 1.5 | |
| Andrena lepida Schenck, 1861 | DD | 1 | 14 | 0 | 15 | 1.0 | |
| Andrena nigroaenea (Kirby, 1802) | LC | 0 | 3 | 0 | 3 | 0.2 | |
| Andrena nilotica Warncke, 1967 | DD | 0 | 1 | 0 | 1 | 0.1 | |
| Andrena ovatula (Kirby, 1802) | NT | 9 | 3 | 0 | 12 | 0.8 | |
| Andrena pilipes Fabricius, 1781 | LC | 1 | 3 | 1 | 5 | 0.3 | |
| Andrena senecionis Pérez, 1895 | LC | 8 | 14 | 2 | 24 | 1.5 | |
| Andrena tenuistriata Pérez, 1895 | LC | 5 | 18 | 1 | 24 | 1.5 | |
| Andrena thoracica (Fabricius, 1775) | DD | 0 | 1 | 0 | 1 | 0.1 | |
| Andrena verticalis Pérez, 1895 | LC | 0 | 10 | 1 | 11 | 0.7 | |
| Panurgus calcaratus (Scopoli, 1763) | LC | 13 | 3 | 2 | 18 | 1.2 | |
| Panurgus cephalotes Latreille, 1811 | LC | 13 | 14 | 6 | 33 | 2.1 | |
| Apidae | Amegilla albigena (Lepeletier, 1841) | LC | 1 | 0 | 0 | 1 | 0.1 |
| Amegilla quadrifasciata (de Villers, 1789) | LC | 1 | 0 | 0 | 1 | 0.1 | |
| Apis mellifera Linnaeus, 1758 | DD | 245 | 285 | 230 | 760 | 48.7 | |
| Ceratina cucurbitina (Rossi, 1792) | LC | 0 | 2 | 0 | 2 | 0.1 | |
| Ceratina cyanea (Kirby, 1802) | LC | 0 | 0 | 1 | 1 | 0.1 | |
| Eucera elongatula Vachal, 1907 | DD | 9 | 10 | 0 | 19 | 1.2 | |
| Eucera notata Lepeletier, 1841 | DD | 81 | 115 | 52 | 248 | 15.9 | |
| Colletidae | Colletes abeillei Pérez, 1903 | LC | 1 | 0 | 0 | 1 | 0.1 |
| Colletes dusmeti Noskiewicz, 1936 | LC | 0 | 1 | 0 | 1 | 0.1 | |
| Hylaeus taeniolatus Förster, 1871 | LC | 0 | 2 | 2 | 4 | 0.3 | |
| Hylaeus variegatus (Fabricius, 1798) | LC | 0 | 1 | 0 | 1 | 0.1 | |
| Halictidae | Halictus fulvipes (Klug, 1817) | LC | 1 | 1 | 0 | 2 | 0.1 |
| Halictus gemmeus Dours, 1872 | LC | 1 | 3 | 1 | 5 | 0.3 | |
| Halictus subauratus (Rossi, 1792) | LC | 1 | 1 | 0 | 2 | 0.1 | |
| Halictus vestitus Lepeletier, 1841 | LC | 1 | 1 | 1 | 3 | 0.2 | |
| Lasioglossum albocinctum (Lucas, 1846) | LC | 6 | 1 | 1 | 8 | 0.5 | |
| Lasioglossum callizonium (Pérez, 1895) | LC | 1 | 2 | 0 | 3 | 0.2 | |
| Lasioglossum discus (Smith, 1853) | LC | 1 | 3 | 1 | 5 | 0.3 | |
| Lasioglossum interruptum (Panzer, 1798) | LC | 39 | 51 | 4 | 94 | 6.0 | |
| Lasioglossum leucozonium (Schrank, 1781) | LC | 4 | 2 | 0 | 6 | 0.4 | |
| Lasioglossum malachurum (Kirby, 1802) | LC | 10 | 34 | 23 | 67 | 4.3 | |
| Lasioglossum mandibulare (Morawitz, 1866) | NT | 4 | 1 | 2 | 7 | 0.4 | |
| Lasioglossum minutissimum (Kirby, 1802) | LC | 0 | 5 | 0 | 5 | 0.3 | |
| Lasioglossum parvulum (Schenck 1853) | LC | 3 | 0 | 2 | 5 | 0.3 | |
| Lasioglossum pauxillum (Schenck, 1853) | LC | 3 | 1 | 5 | 9 | 0.6 | |
| Lasioglossum villosulum (Kirby, 1802) | LC | 2 | 0 | 1 | 3 | 0.2 | |
| Lasioglossum virens (Erichson, 1835) | EN | 0 | 1 | 1 | 2 | 0.1 | |
| Nomioides minutissimus (Rossi, 1790) | LC | 0 | 0 | 1 | 1 | 0.1 | |
| Ceylalictus variegatus (Olivier, 1789) | LC | 0 | 0 | 1 | 1 | 0.1 | |
| Megachilidae | Anthidium punctatum Latreille, 1809 | LC | 0 | 0 | 1 | 1 | 0.1 |
| Anthidium taeniatum Latreille, 1809 | DD | 0 | 1 | 0 | 1 | 0.1 | |
| Hoplitis acuticornis (Dufour & Perris, 1840) | LC | 1 | 0 | 0 | 1 | 0.1 | |
| Hoplitis adunca (Panzer, 1798) | LC | 0 | 1 | 0 | 1 | 0.1 | |
| Hoplitis ochraceicornis (Ferton, 1902) | LC | 1 | 1 | 0 | 2 | 0.1 | |
| Hoplitis papaveris (Latreille, 1799) | LC | 1 | 3 | 0 | 4 | 0.3 | |
| Osmia aurulenta Panzer, 1799 | LC | 1 | 1 | 0 | 2 | 0.1 | |
| Osmia ferruginea Latreille, 1811 | LC | 1 | 0 | 0 | 1 | 0.1 | |
| Osmia latreillei (Spinola, 1806) | LC | 1 | 0 | 0 | 1 | 0.1 | |
| Osmia tricornis Latreille, 1811 | LC | 1 | 0 | 0 | 1 | 0.1 | |
| Osmia niveata (Fabricius 1804) | LC | 0 | 1 | 0 | 1 | 0.1 | |
| Rhodanthidium infuscatum (Erichson, 1835) | DD | 1 | 0 | 0 | 1 | 0.1 | |
| Rhodanthidium sticticum (Fabricius, 1787) | DD | 3 | 0 | 0 | 3 | 0.2 | |
| Melittidae | Dasypoda cingulata Erichson, 1835 | LC | 1 | 0 | 0 | 1 | 0.1 |
| Bee Group | Plant Family | Coefficient | SE | x2-Value | df | p-Value |
|---|---|---|---|---|---|---|
| A. mellifera | Apiaceae | −191.1 | 136.8 | 2.031 | 1 | 0.1541 |
| Asteraceae | −2.925 | 1.195 | 6.234 | 1 | 0.0125 | |
| Boraginaceae | 3.699 | 0.343 | 120.8 | 1 | <0.001 | |
| Brassicaceae | 1.987 | 0.232 | 76.58 | 1 | <0.001 | |
| Fabaceae | 0.593 | 1.123 | 0.291 | 1 | 0.5899 | |
| Lamiaceae | 2.798 | 0.663 | 18.54 | 1 | <0.001 | |
| Megachilidae | Apiaceae | −2.630 | 2.321 | 1.336 | 1 | 0.2477 |
| Asteraceae | −3.287 | 3.445 | 0.947 | 1 | 0.3305 | |
| Boraginaceae | 0.701 | 1.062 | 0.454 | 1 | 0.5004 | |
| Brassicaceae | 0.456 | 0.987 | 0.222 | 1 | 0.6375 | |
| Fabaceae | 0.905 | 1.578 | 0.342 | 1 | 0.5586 | |
| Lamiaceae | 2.922 | 1.521 | 3.842 | 1 | 0.0500 | |
| Mining bees | Apiaceae | 0.831 | 0.832 | 1.038 | 1 | 0.3083 |
| Asteraceae | 0.581 | 1.135 | 0.273 | 1 | 0.6014 | |
| Boraginaceae | 1.148 | 0.504 | 5.395 | 1 | 0.0202 | |
| Brassicaceae | 0.977 | 0.444 | 5.033 | 1 | 0.0249 | |
| Fabaceae | 0.257 | 1.547 | 0.029 | 1 | 0.8654 | |
| Lamiaceae | 0.707 | 1.576 | 0.210 | 1 | 0.6471 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez, J.A.; Carrasco, A.; La Spina, M.; Pérez-Marcos, M.; Ortiz-Sánchez, F.J. How Bees Respond Differently to Field Margins of Shrubby and Herbaceous Plants in Intensive Agricultural Crops of the Mediterranean Area. Insects 2020, 11, 26. https://doi.org/10.3390/insects11010026
Sanchez JA, Carrasco A, La Spina M, Pérez-Marcos M, Ortiz-Sánchez FJ. How Bees Respond Differently to Field Margins of Shrubby and Herbaceous Plants in Intensive Agricultural Crops of the Mediterranean Area. Insects. 2020; 11(1):26. https://doi.org/10.3390/insects11010026
Chicago/Turabian StyleSanchez, Juan Antonio, Aline Carrasco, Michelangelo La Spina, María Pérez-Marcos, and F. Javier Ortiz-Sánchez. 2020. "How Bees Respond Differently to Field Margins of Shrubby and Herbaceous Plants in Intensive Agricultural Crops of the Mediterranean Area" Insects 11, no. 1: 26. https://doi.org/10.3390/insects11010026
APA StyleSanchez, J. A., Carrasco, A., La Spina, M., Pérez-Marcos, M., & Ortiz-Sánchez, F. J. (2020). How Bees Respond Differently to Field Margins of Shrubby and Herbaceous Plants in Intensive Agricultural Crops of the Mediterranean Area. Insects, 11(1), 26. https://doi.org/10.3390/insects11010026