Molecular Phylogeny and Infraordinal Classification of Zoraptera (Insecta)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Taxon Sampling and Laboratory Methods
2.2. Dataset Assembly and Phylogenetic Analyses
2.3. Morphology
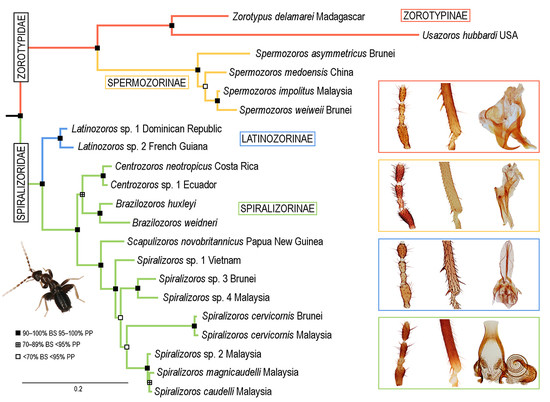
3. Results
3.1. Phylogenetic Analyses
3.2. Infraordinal Classification and Taxonomy
3.2.1. Family Zorotypidae Silvestri, 1913, stat. revid.
Subfamily Zorotypinae Silvestri, 1913
Genus Zorotypus Silvestri, 1913, stat. revid.
Usazoros Kukalova-Peck and Peck, 1993, stat. restit.
Subfamily Spermozorinae subfam. nov.
Genus Spermozoros gen. nov.
3.2.2. Family Spiralizoridae fam. nov.
Subfamily Latinozorinae subfam. nov.
Genus Latinozoros Kukalova-Peck and Peck, 1993, stat. restit.
Subfamily Spiralizorinae subfam. nov.
Genus Spiralizoros gen. nov.
Centrozoros Kukalova-Peck and Peck, 1993, stat. restit.
Brazilozoros Kukalova-Peck and Peck, 1993, stat. restit.
Cordezoros gen. nov.
Scapulizoros gen. nov.
3.2.3. Species Incertae Sedis in Zoraptera
Zorotypus congensis van Ryn Tournel, 1971
Zorotypus javanicus Silvestri, 1913
Zorotypus juninensis Engel, 2000
Zorotypus lawrencei New, 1995
Zorotypus leleupi Weidner, 1976
Zorotypus longicercatus Caudell, 1927
Zorotypus newi (Chao and Chen, 2000)
Zorotypus sechellensis Zompro, 2005
Zorotypus swezeyi Caudell, 1922
3.3. Generic Identification Key (Based on Males)
- 1.
- -
- 2.
- -
- 3.
- Ventral edge of metatibia with three stout spurs (Figure 2F,J); mating hook on abdominal tergite X well developed, prolonged. Neotropical/Afrotropical regions..........................................................................................Zorotypus Silvestri, 1913, stat. revid.
- -
- Ventral edge of metatibia with three inconspicuous spurs (Figure 2H); mating hook absent, tergites X and XI with small median protuberance. Nearctic region...........................................................................Usazoros Kukalova-Peck and Peck, 1993, stat. restit.
- 4.
- Metatibia with two stout spurs on ventral surface, of which only one is located apically (Figure 4C); antennomere I approximately as long as antennomeres II and III combined (Figure 4A). Abdominal tergites X and XI with median projections. Panamanian and Neotropical regions............................................................Latinozoros Kukalova-Peck and Peck, 1993, stat. restit.
- -
- Metatibia with two small apical spurs or spurs absent (Figure 5J,K); antennomere I approximately as long as antennomere III (Figure 5B); abdominal tergite X flat, abdominal tergite XI with median projection (Figure 5S,T)...........................................................................................................................5
- 5.
- Elongated intromittent organ present (Figure 5L,M,O,R)................................................................................................................6
- -
- Elongated intromittent organ absent (Figure 5P). Neotropical region.............................................Brazilozoros Kukalova-Peck and Peck, 1993, stat. restit.
- 6.
- Intromittent organ coiled (Figure 5L,M,R)..........................................................7
- -
- Intromittent organ dorsoventrally looped (Figure 5O). Oceanian region........................................................................................................................8
- 7.
- Basal plate of genitalia anteriorly strongly dilated, with diverging sides (Figure 5L–N). Oriental region............................................................Spiralizoros gen. nov.
- -
- Basal plate of genitalia not dilated anteriorly, with sides converging anteriorly (Figure 5R). Nearctic-Panamanian-Neotropical regions..............................Centrozoros gen. nov.
- 8.
- Distal half of metafemur with row of short stout bristles on ventral surface (Figure 5I). Male genitalia with strongly sclerotized heart-shaped sclerite (Figure 5O). Papua New Guinea........................................................................................................................Cordezoros gen. nov.
- -
- Distal half of metafemur with row of long stout bristles on ventral surface (Figure 5H). Male genitalia without heart-shaped sclerite. Fiji..............................Scapulizoros gen. nov.
4. Discussion
4.1. Phylogeny and Classification of Zoraptera
4.2. Reproduction Strategies in Zorotypidae and Spiralizoridae
4.3. Diversity and Distribution of Extant Zoraptera
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mashimo, Y.; Matsumura, Y.; Machida, R.; Dallai, R.; Gottardo, M.; Yoshizawa, K.; Friedrich, F.; Wipfler, B.; Beutel, R.G. 100 years Zoraptera—A phantom in insect evolution and the history of its investigation. Insect Syst. Evol. 2014, 45, 371–393. [Google Scholar] [CrossRef] [Green Version]
- Choe, J.C. Biodiversity of Zoraptera and their little-known biology. In Insect Biodiversity: Science and Society, 1st ed.; Foottit, R.G., Adler, P.H., Eds.; John Wiley & Sons Ltd.: New York, NY, USA, 2018; Volume 2, pp. 199–217. [Google Scholar] [CrossRef]
- Evangelista, D.A.; Wipfler, B.; Béthoux, O.; Donath, A.; Fujita, M.; Kohli, M.K.; Legendre, F.; Liu, S.; Machida, R.; Misof, B.; et al. An integrative phylogenomic approach illuminates the evolutionary history of cockroaches and termites (Blattodea). Proc. R. Soc. B 2019, 286, 20182076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montagna, M.; Tong, K.J.; Magoga, G.; Strada, L.; Tintori, A.; Ho, S.Y.; Lo, N. Recalibration of the insect evolutionary time scale using Monte San Giorgio fossils suggests survival of key lineages through the End-Permian Extinction. Proc. R. Soc. B 2019, 286, 20191854. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Beutel, R.G.; Rafael, J.A.; Yao, I.; Camara, J.T.; Lima, S.P.; Yoshizawa, K. The evolution of Zoraptera. Syst. Entomol. 2019. [Google Scholar] [CrossRef] [Green Version]
- Beutel, R.G.; Weide, D. Cephalic anatomy of Zorotypus hubbardi (Hexapoda: Zoraptera): New evidence for a relationship with Acercaria. Zoomorphology 2005, 124, 121–136. [Google Scholar] [CrossRef]
- Misof, B.; Liu, S.; Meusemann, K.; Peters, R.S.; Donath, A.; Mayer, C.; Frandsen, P.B.; Ware, J.; Flouri, T.; Beutel, R.G.; et al. Phylogenomics resolves the timing and pattern of insect evolution. Science 2014, 346, 763–767. [Google Scholar] [CrossRef]
- Wipfler, B.; Letsch, H.; Frandsen, P.B.; Kapli, P.; Mayer, C.; Bartel, D.; Buckley, T.R.; Donath, A.; Edgerly-Rooks, J.S.; Fujita, M.; et al. Evolutionary history of Polyneoptera and its implications for our understanding of early winged insects. Proc. Natl. Acad. Sci. 2019, 116, 3024–3029. [Google Scholar] [CrossRef] [Green Version]
- Chesters, D. The phylogeny of insects in the data-driven era. Syst. Entomol. 2019. [Google Scholar] [CrossRef]
- Terry, M.D.; Whiting, M.F. Mantophasmatodea and phylogeny of the lower neopterous insects. Cladistics 2005, 21, 240–257. [Google Scholar] [CrossRef]
- Silvestri, F. Descrizione di un nuovo ordine di insetti. Boll. Lab. Zool. Gen. Agrar. Portici 1913, 7, 193–209. [Google Scholar]
- Mashimo, Y.; Matsumura, Y.; Beutel, R.G.; Njoroge, L.; Machida, R. A remarkable new species of Zoraptera, Zorotypus asymmetristernum sp. n., from Kenya (Insecta, Zoraptera, Zorotypidae). Zootaxa 2018, 4388, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Kukalova-Peck, J.; Peck, S.B. Zoraptera wing structures: Evidence for new genera and relationship with the blattoid orders (Insecta: Blattoneoptera). Syst. Entomol. 1993, 18, 333–350. [Google Scholar] [CrossRef]
- Chao, R.F.; Chen, C.S. Formosozoros newi, a new genus and species of Zoraptera (Insecta) from Taiwan. Pan-Pac. Entomol. 2000, 76, 24–27. [Google Scholar]
- Engel, M.S.; Grimaldi, D.A. A winged Zorotypus in Miocene amber from the Dominican Republic (Zoraptera: Zorotypidae), with discussion on relationships of and within the order. Acta Geol. Hisp. 2000, 35, 149–164. [Google Scholar]
- Engel, M.S. Phylogeny of the Zoraptera. Entomol. Abh. 2003, 61, 147–148. [Google Scholar]
- Engel, M.S.; Grimaldi, D.A. The first Mesozoic Zoraptera (Insecta). Am. Mus. Novit. 2002, 3362, 1–20. [Google Scholar] [CrossRef]
- Hünefeld, F. The genital morphology of Zorotypus hubbardi Caudell, 1918 (Insecta: Zoraptera: Zorotypidae). Zoomorphology 2007, 126, 135–151. [Google Scholar] [CrossRef]
- Dallai, R.; Mercati, D.; Gottardo, M.; Dossey, A.T.; Machida, R.; Mashimo, Y.; Beutel, R.G. The male and female reproductive systems of Zorotypus hubbardi Caudell, 1918 (Zoraptera). Arthropod Struct. Dev. 2012, 41, 337–359. [Google Scholar] [CrossRef]
- Dallai, R.; Gottardo, M.; Mercati, D.; Machida, R.; Mashimo, Y.; Matsumura, Y.; Rafael, J.A.; Beutel, R.G. Comparative morphology of spermatozoa and reproductive systems of zorapteran species from different world regions (Insecta, Zoraptera). Arthropod Struct. Dev. 2014, 43, 371–383. [Google Scholar] [CrossRef]
- Dallai, R.; Gottardo, M.; Mercati, D.; Rafael, J.A.; Machida, R.; Mashimo, Y.; Matsumutra, Y.; Beutel, R.G. The intermediate sperm type and genitalia of Zorotypus shannoni Gurney: Evidence supporting infraordinal lineages in Zoraptera (Insecta). Zoomorphology 2015, 134, 79–91. [Google Scholar] [CrossRef]
- Boudinot, B.E. A general theory of genital homologies for the Hexapoda (Pancrustacea) derived from skeletomuscular correspondences, with emphasis on the Endopterygota. Arthropod Struct. Dev. 2018, 47, 563–613. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, K.; Johnson, K.P. Aligned 18S for Zoraptera (Insecta): Phylogenetic position and molecular evolution. Mol. Phylogenet. Evol. 2005, 37, 572–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, C.; Wang, Y.; Wu, C.; Kang, L.; Liu, C. The compact mitochondrial genome of Zorotypus medoensis provides insights into phylogenetic position of Zoraptera. BMC Genom. 2014, 15, 1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colgan, D.J.; Cassis, G.; Beacham, E. Setting the molecular phylogenetic framework for the Dermaptera. Insect. Syst. Evol. 2003, 34, 65–80. [Google Scholar] [CrossRef]
- Kamimura, Y. In search of the origin of twin penises: Molecular phylogeny of earwigs (Dermaptera: Forficulina) based on mitochondrial and nuclear ribosomal RNA genes. Ann. Entomol. Soc. Am. 2004, 97, 903–912. [Google Scholar] [CrossRef] [Green Version]
- Jarvis, K.J.; Haas, F.; Whiting, M.F. Phylogeny of earwigs (Insecta: Dermaptera) based on molecular and morphological evidence: Reconsidering the classification of Dermaptera. Syst. Entomol. 2005, 30, 442–453. [Google Scholar] [CrossRef]
- Kočárek, P.; John, V.; Hulva, P. When the body hides the ancestry: Phylogeny of morphologically modified epizoic earwigs based on molecular evidence. PLoS ONE 2013, 8, e66900. [Google Scholar] [CrossRef] [Green Version]
- Kirstová, M.; Kundrata, R.; Kočárek, P. Molecular phylogeny and classification of Chelidurella Verhoeff, stat. restit. (Dermaptera: Forficulidae). Insect Syst. Evol. in press.
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [CrossRef] [Green Version]
- Crandall, K.A.; Fitzpatrick, J.F., Jr. Crayfish molecular systematics: Using a combination of procedures to estimate phylogeny. Syst. Biol. 1996, 45, 1–26. [Google Scholar] [CrossRef]
- Whiting, M.F.; Carpenter, J.C.; Wheeler, Q.D.; Wheeler, W.C. The Strepsiptera problem: Phylogeny of the holometabolous insect orders inferred from 18S and 28S ribosomal DNA sequences and morphology. Syst. Biol. 1997, 46, 1–68. [Google Scholar] [CrossRef] [PubMed]
- Palumbi, S.; Martin, A.; Romaro, S.; McMillan, W.O.; Stice, L.; Grabowski, G. The Simple Fool’s Guide to PCR. Version 2.0; University of Hawaii: Honolulu, HI, USA, 2002; pp. 1–45. [Google Scholar]
- Machida, R.J.; Knowlton, N. PCR Primers for metazoan nuclear 18S and 28S ribosomal DNA sequences. PLoS ONE 2012, 7, e46180. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanfear, R.; Calcott, B.; Ho, S.Y.W.; Guindon, S. PartitionFinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 2012, 29, 1695–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010. [Google Scholar]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A.; Hoover, P.; Rougemont, J. A rapid bootstrap algorithm for the RAxML web servers. Syst. Biol. 2008, 57, 758–771. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [Green Version]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.; Xie, Z.; Salemi, M.; Chen, L.; Wang, Y. An index of substitution saturation and its application. Mol. Phylogenet. Evol. 2003, 26, 1–7. [Google Scholar] [CrossRef]
- Xia, X.; Lemey, P. Assessing substitution saturation with DAMBE. In The Phylogenetic Handbook: A Practical Approach to DNA and Protein Phylogeny, 2nd ed.; Lemey, P., Salemi, M., Vandamme, A.M., Eds.; Cambridge University Press: Cambridge, UK, 2009; pp. 615–630. [Google Scholar]
- Kočárek, P.; Wahab, R.A.; Abdul Kahar, S.R. Zorotypus asymmetricus sp. nov. from Brunei Darussalam, Borneo (Insecta: Zoraptera). Zootaxa 2017, 4286, 285–290. [Google Scholar] [CrossRef] [Green Version]
- Engel, M.S. A new apterous Zorotypus in Miocene amber from the Dominican Republic (Zoraptera: Zorotypidae). Acta Entomol. Slov. 2008, 16, 127–136. [Google Scholar]
- Holt, B.G.; Lessard, J.-P.; Borregaard, M.K.; Fritz, S.A.; Araújo, M.B.; Dimitrov, D.; Fabre, P.-H.; Graham, C.H.; Graves, G.R.; Jønsson, K.A.; et al. An update of Wallace’s zoogeographic regions of the World. Science 2013, 339, 74–78. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Engel, M.S.; Rafael, J.A.; Dang, K.; Wu, H.; Wang, Y.; Xie, Q.; Bu, W.A. Unique box in 28S rRNA is shared by the enigmatic insect order Zoraptera and Dictyoptera. PLoS ONE 2013, 8, E53679. [Google Scholar] [CrossRef] [Green Version]
- Gurney, A.B. A synopsis of the order Zoraptera, with notes on the biology of Zorotypus hubbardi Caudell. Proc. Entomol. Soc. Wash. 1938, 40, 57–87. [Google Scholar]
- New, T.R. Notes on Neotropical Zoraptera, with descriptions of two new species. Syst. Entomol. 1978, 3, 361–370. [Google Scholar] [CrossRef]
- Rafael, J.A.; Engel, M.S. A new species of Zorotypus from central Amazonia, Brazil (Zoraptera: Zorotypidae). Am. Mus. Novit. 2006, 3528, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Rafael, J.A.; Godoi, F.S.; Engel, M.S. A new species of Zorotypus from eastern Amazonia, Brazil (Zoraptera: Zorotypidae). Trans. Kans. Acad. Sci. 2008, 111, 193–202. [Google Scholar] [CrossRef]
- Yin, Z.W.; Li, L.Z. Zorotypus huangi sp. nov. (Zoraptera: Zorotypidae) from Yunnan, southern China. Zootaxa 2017, 4300, 287–294. [Google Scholar] [CrossRef]
- Mashimo, Y.; Yoshizawa, K.; Engel, M.S.; Ghani, I.A.; Dallai, R.; Beutel, R.G.; Machida, R. Zorotypus in Peninsular Malaysia (Zoraptera: Zorotypidae), with the description of three new species. Zootaxa 2013, 3717, 498–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Li, H.; Cai, W. Zorotypus weiweii (Zoraptera: Zorotypidae), a new species of angel insects, from Sabah, East Malasia. Zootaxa 2016, 4162, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.S. Zorotypus chinensis, a new species from China. Acta Entomol. Sin. 1974, 17, 423–427, (In Chinese, English summary). [Google Scholar]
- Karny, H.H. Spolia Mentawiensia. Zoraptera. Treubia 1927, 9, 1–5. [Google Scholar]
- New, T.R. Zoraptera (Insecta) in east Malaysia: Notes on Zorotypus caudelli Karny. Orient. Insects 2000, 34, 77–82. [Google Scholar] [CrossRef]
- Karny, H.H. Zorapteren aus Süd-Sumatra. Treubia 1922, 3, 14–29. [Google Scholar]
- Karny, H.H. Psocoptera. Insects of Samoa and Other Samoan Terrestrial Arthropoda. Part VII. Other Orders of Insects; British Museum (Natural History): London, UK, 1932; pp. 117–129. [Google Scholar]
- Choe, J.C. Zorotypus gurneyi, new species, from Panama and redescription of Z. barberi Gurney (Zoraptera: Zorotypidae). Ann. Entomol. Soc. Am. 1989, 82, 149–155. [Google Scholar] [CrossRef]
- Choe, J.C. Zoraptera of Panama with a review of the morphology, systematics, and biology of the order. In Insects of Panama and Mesoamerica: Selected Studies; Quintero, D., Aiello, A., Eds.; Oxford University Press: Oxford, UK, 1992; pp. 249–256. [Google Scholar]
- Caudell, A.N. A new species of Zoraptera from Bolivia. Proc. Entomol. Soc. Wash. 1923, 25, 60–62. [Google Scholar]
- Bolívar y Pieltain, C.; Coronado, G.L. Estudio de un nuevo Zorotypus proveniente de la Region Amazonica Peruana (Ins., Zoraptera). Ciencia 1963, 22, 93–100. [Google Scholar]
- Silvestri, F. Descrizione di due specie neotropicali di Zorotypus (Insecta, Zoraptera). Boll. Lab. Entomol. Agrar. Portici 1946, 7, 1–12. [Google Scholar]
- Gurney, A.B. A new species of Zoraptera from Fiji. Occas. Pap. Bernice P. Bishop Mus. 1939, 15, 161–165. [Google Scholar]
- Terry, M.D.; Whiting, M.F. Zorotypus novobritannicus n. sp., the first species of the order Zoraptera (Zorotypidae) from the Australasian Ecozone. Zootaxa 2012, 3260, 53–61. [Google Scholar] [CrossRef]
- Van Ryn-Tournel, J. Presence de l’ordre des Zorapthres au Congo et description de Zorotypus congensis sp. n. Rev. Zool. Bot. Afr. 1971, 83, 100–110. [Google Scholar]
- Engel, M.S. A new Zorotypus from Peru, with notes on related neotropical species (Zoraptera: Zorotypidae). J. Kans. Entomol. Soc. 2000, 73, 11–20. [Google Scholar]
- New, T.R. The order Zoraptera (Insecta) from Christmas Island, Indian Ocean. Invertebr. Syst. 1995, 9, 243–246. [Google Scholar] [CrossRef]
- Weidner, H. Eine neue Zorotypus-Art von den Galapagosinseln, Zorotypus leleupi sp.n. (Zoraptera). In Mission Zoologique Belge aux iles Galapagos et en Ecuador (N. et J. Leleup 1964–1965); Institut Royal des Sciences Naturelles de Belgique: Bruxelles, Belgium, 1976; pp. 161–176. [Google Scholar]
- Caudell, A.N. Zorotypus longicercatus, a new species of Zoraptera from Jamaica. Proc. Entomol. Soc. Wash. 1927, 29, 144–145. [Google Scholar]
- Zompro, O. Inter- and intra-ordinal relationships of the Mantophasmatodea, with comments on the phylogeny of polyneopteran orders (Insecta: Polyneoptera). Mitt. Geol-Paläont. Inst. Univ. Hambg. 2005, 89, 85–116. [Google Scholar]
- Caudell, A.N. Zorotypus swezeyi, a new species of the order Zoraptera from Hawaii. Trans. Am. Entomol. Soc. 1922, 48, 133–135. [Google Scholar]
- Matsumura, Y.; Yoshizawa, K.; Machida, R.; Mashimo, Y.; Dallai, R.; Gottardo, M.; Kleinteich, T.; Michels, J.; Gorb, S.N.; Beutel, R.G. Two intromittent organs in Zorotypus caudelli (Insecta, Zoraptera): The paradoxical coexistence of an extremely long tube and a large spermatophore. Zool. J. Linn. Soc. 2014, 112, 40–54. [Google Scholar] [CrossRef] [Green Version]
- Yin, Z.; Cai, C.; Huang, D. New zorapterans (Zoraptera) from Burmese amber suggest higher paleodiversity of the order in tropical forests. Cretac. Res. 2018, 84, 168–172. [Google Scholar] [CrossRef]
- Yin, Z.; Cai, C.; Huang, D.; Engel, M.S. Zorotypus dilaticeps sp. nov., a remarkable zorapteran (Zoraptera) in mid-Cretaceous Burmese amber. Cretac. Res. 2018, 91, 126–130. [Google Scholar] [CrossRef]
- Mashimo, Y.; Mueller, P.; Pohl, H.; Beutel, R.G. The “hairy beast”-Zorotypus hirsutus sp. n., an unusual new species of Zoraptera (Insecta) from Burmese amber. Zootaxa 2018, 4508, 562–568. [Google Scholar] [CrossRef]
- Mashimo, Y.; Mueller, P.; Beutel, R.G. Zorotypus pecten, a new species of Zoraptera (Insecta) from mid-Cretaceous Burmese amber. Zootaxa 2019, 4651, 565–577. [Google Scholar] [CrossRef]
- Chen, X.; Su, G. A new species of Zorotypus (Insecta, Zoraptera, Zorotypidae) and the earliest known suspicious mating behavior of Zorapterans from the mid-cretaceous amber of northern Myanmar. J. Zool. Syst. Evol. Res. 2019, 57, 555–560. [Google Scholar] [CrossRef] [Green Version]
- Dallai, R.; Gottardo, M.; Mercati, D.; Machida, R.; Mashimo, Y.; Beutel, R.G. Divergent mating patterns and a unique mode of external sperm transfer in Zoraptera: An enigmatic group of pterygote insects. Naturwissenschaften 2013, 100, 581–594. [Google Scholar] [CrossRef]
- Choe, J.C. Sexual selection and mating system in Zorotypus gurneyi Choe (Insecta: Zoraptera): I. Dominance hierarchy and mating success. Behav. Ecol. Sociobiol. 1994, 34, 87–93. [Google Scholar] [CrossRef]
- Choe, J.C. Courtship feeding and repeated mating in Zorotypus barberi (Insecta: Zoraptera). Anim. Behav. 1995, 6, 1511–1520. [Google Scholar] [CrossRef]
- Choe, J.C. The evolution of mating systems in the Zoraptera: Mating variations and sexual conflicts. In The Evolution of Mating Systems in Insects and Arachnids; Choe, J.C., Crespi, B.J., Eds.; Cambridge University Press: Cambridge, UK, 1997; pp. 130–145. [Google Scholar] [CrossRef]
- Shetlar, D.J. Biological observations on Zorotypus hubbardi Caudell (Zoraptera). Entomol. News 1978, 89, 217–223. [Google Scholar]
- Hubbard, M.D. A catalog of the order Zoraptera (Insecta). Insecta Mundi 1990, 4, 49–66. [Google Scholar]
- Riegel, G.T. The distribution of Zorotypus hubbardi (Zoraptera). Ann. Entomol. Soc. Am. 1963, 56, 744–747. [Google Scholar] [CrossRef]
- Grimaldi, D.; Engel, M.S. Evolution of the Insects, 1st ed.; Cambridge University Press: Cambridge, UK, 2005; p. 755. [Google Scholar]





| Order/Family | Genus/Species | Voucher Number | Geographic Origin | 18S rRNA | 16S rRNA | COI mtDNA |
|---|---|---|---|---|---|---|
| Dermaptera | ||||||
| Forficulidae | Forficula auricularia | 014 | Czech Republic | MN790613 | MN790594 | MN790630 |
| Forficulidae | Anechura bipunctata | 065 | Mongolia | MN790616 | MN790596 | MH853426 1 |
| Forficulidae | Chelidurella acanthopygia | 002/016 * | Czech Republic | MN790612 | MN790593 | MH853444 1 |
| Anisolabididae | Euborellia arcanum | E8 | USA | MN790614 | MN790595 | MH853422 1 |
| Diplatyidae | Schizodiplatys sp. | 084 | Brunei | MN790610 | N/A | MN790628 |
| Pyginicranidae | Pyragropsis thoracica | 077 | French Guiana | MN790611 | N/A | MN790629 |
| Pyginicranidae | Cranopygia sp. | 081 | Brunei | MN790615 | N/A | MN790631 |
| Zoraptera | ||||||
| Zorotypidae | Zorotypus delamarei | 71Z | Madagascar | MN790597 | MN790583 | MN790618 |
| Zorotypidae | Usazoros hubbardi | Zohub | USA | DQ013288 2 | N/A | N/A |
| Zorotypidae | Spermozoros asymmetricus | 4Z | Brunei | MN790598 | MN790592 | MN790617 |
| Zorotypidae | Spermozoros impolitus | 68Z | Malaysia | MN790601 | N/A | N/A |
| Zorotypidae | Spermozoros weiweii | 19Z | Brunei | MN790602 | MN790591 | MN790622 |
| Zorotypidae | Spermozoros medoensis | - | China | KM246626 3 | JQ910991 3 | KJ467512 3 |
| Spiralizoridae | Spiralizoros sp. 1 | KY330 (VN) | Vietnam | DQ013290 2 | N/A | N/A |
| Spiralizoridae | Spiralizoros sp. 2 | KY325 (MY1) | Malaysia | DQ013289 2 | N/A | N/A |
| Spiralizoridae | Spiralizoros caudelli | 44Z | Malaysia | MN790605 | MN790585 | MN790624 |
| Spiralizoridae | Spiralizoros magnicaudelli | 6Z | Malaysia | MN790600 | MN790590 | MN790627 |
| Spiralizoridae | Spiralizoros sp. 3 | 13Z | Brunei | MN790608 | MN790589 | MN790623 |
| Spiralizoridae | Spiralizoros sp. 4 | 8Z | Malaysia | MN790609 | MN790587 | MN790625 |
| Spiralizoridae | Spiralizoros cervicornis | KY328 (MY2) | Malaysia | DQ013291 2 | N/A | N/A |
| Spiralizoridae | Spiralizoros cervicornis | 1Z | Brunei | MN790599 | MN790588 | MN790626 |
| Spiralizoridae | Scapulizoros novobritannicus | BYU ZO002 | Papua New Guinea | AY521891 4 | EF623273 5 | N/A |
| Spiralizoridae | Centrozoros sp. 1 | 38Z | Ecuador | MN790607 | MN790586 | N/A |
| Spiralizoridae | Centrozoros neotropicus | 43Z | Costa Rica | MN790606 | MN790584 | MN790619 |
| Spiralizoridae | Latinozoros sp. 1 | 41Z | Dominican Republic | MN790603 | N/A | MN790621 |
| Spiralizoridae | Latinozoros sp. 2 | 48Z | French Guiana | MN790604 | N/A | MN790620 |
| Spiralizoridae | Brazilozoros huxleyi | - | - | JQ259055 6 | N/A | N/A |
| Spiralizoridae | Brazilozoros weidneri | - | - | JQ259056 6 | N/A | N/A |
| Family | Subfamily | Genus | Species | Distribution |
|---|---|---|---|---|
| Zorotypidae Silvestri, 1913 stat. revid. | Zorotypinae Silvestri, 1913 stat. revid. | Zorotypus Silvestri, 1913, stat. revid. | Zorotypus guineensis Silvestri, 1913 * | Guinea, Ghana, Ivory Coast |
| Zorotypus asymmetristernum Mashimo, 2019 | Kenya | |||
| Zorotypus delamarei Paulian, 1949 | Madagascar | |||
| Zorotypus vinsoni Paulian, 1951 | Mauritius | |||
| Zorotypus shannoni Gurney, 1938 | Brazil | |||
| Zorotypus amazonensis Rafael and Engel, 2006 | Brazil | |||
| Zorotypus caxiuana Rafael, Godoi and Engel, 2008 | Brazil | |||
| Usazoros Kukalova-Peck and Peck, 1993, stat. restit. | Usazoros hubbardi (Caudell, 1918), stat. restit. * | USA | ||
| Spermozorinae subfam. nov. | Spermozoros gen. nov. | Spermozoros asymmetricus (Kočárek, 2017), comb. nov. * | Brunei | |
| Spermozoros huangi (Yin and Li, 2017), comb. nov. | China: Yunnan | |||
| Spermozoros impolitus (Mashimo, Engel, Dallai, Beutel and Machida, 2013), comb. nov. | Peninsular Malaysia | |||
| Spermozoros medoensis (Huang, 1976), comb. nov. | China: Tibet | |||
| Spermozoros sinensis (Huang, 1974), comb. nov. | China: Tibet | |||
| Spermozoros weiweii (Wang, Li and Cai, 2016), comb. nov. | Borneo | |||
| Spiralizoridae fam.nov. | Spiralizorinae subfam. nov. | Spiralizoros gen. nov. | Spiralizoros cervicornis (Mashimo, Yoshizawa and Engel, 2013), comb. nov. * | Peninsular Malaysia, Borneo |
| Spiralizoros buxtoni (Karny, 1932), comb. nov. | Samoa | |||
| Spiralizoros caudelli (Karny, 1922), comb. nov. | Peninsular Malaysia, Sumatra | |||
| Spiralizoros ceylonicus (Silvestri, 1913), comb. nov. | Sri Lanka | |||
| Spiralizoros hainanensis (Yin, Li and Wu, 2015), comb. nov. | China: Hainan | |||
| Spiralizoros magnicaudelli (Mashimo, Engel, Dallai, Beutel and Machida, 2013), comb. nov. | Peninsular Malaysia, Borneo | |||
| Spiralizoros philippinensis (Gurney, 1938), comb. nov. | Philippines | |||
| Spiralizoros silvestrii (Karny, 1927), comb. nov. | Indonesia: Mentawai Islands | |||
| Spiralizoros sp. 1 | Vietnam [23] | |||
| Spiralizoros sp. 2 | Malaysia [23] | |||
| Spiralizoros sp. 3 | Brunei (unpublished) | |||
| Spiralizoros sp. 4 | Borneo (unpublished) | |||
| Centrozoros Kukalova-Peck and Peck, 1993, stat. restit. | Centrozoros gurneyi (Choe, 1989), stat. restit. * | Costa Rica, Panama | ||
| Centrozoros cramptoni (Gurney, 1938), comb. nov. | Guatemala | |||
| Centrozoros snyderi (Caudell, 1920), comb. nov. | USA; Jamaica | |||
| Centrozoros hamiltoni (New, 1978), comb. nov. | Colombia, Barbados | |||
| Centrozoros manni (Caudell, 1923) comb. nov. | Bolivia, Peru | |||
| Centrozoros mexicanus (Bolívar y Pieltain, 1940), comb. nov. | Mexico | |||
| Centrozoros neotropicus (Silvestri, 1916), comb. nov. | Costa Rica | |||
| Centrozoros sp. 1 | Ecuador (unpublished) | |||
| Brazilozoros Kukalova-Peck and Peck, 1993, stat. restit. | Brazilozoros brasiliensis (Silvestri, 1946), stat. restit. * | Brazil | ||
| Brazilozoros weidneri (New, 1978), comb. nov. | Brazil | |||
| Brazilozoros huxleyi (Bolívar y Pieltain and Coronado, 1963), comb. nov. | Brazil, Peru, Guyana, Ecuador | |||
| Scapulizoros gen. nov. | Scapulizoros novobritannicus (Terry and Whiting, 2012), comb. nov. * | Papua New Guinea | ||
| Cordezoros gen. nov. | Cordezoros zimmermani (Gurney, 1939), comb. nov. * | Fiji | ||
| Latinozorinae subfam. nov. | Latinozoros Kukalova-Peck and Peck, 1993, stat. restit. | Latinozoros barberi (Gurney, 1938), stat. restit. * | Panama, Costa Rica, Venezuela (?), Trinidad and Tobago (?), Puerto Rico (?) | |
| Latinozoros sp. 1 | Dominican Republic (unpublished) | |||
| Latinozoros sp. 2 | French Guiana (unpublished) | |||
| Zoraptera incertae sedis | Zorotypus congensis van Ryn Tournel, 1971 | Congo | ||
| Zorotypus javanicus Silvestri, 1913 | Indonesia: Java | |||
| Zorotypus juninensis Engel, 2000 | Peru | |||
| Zorotypus lawrencei New, 1995 | Christmas Island | |||
| Zorotypus leleupi Weidner, 1976 | Galapagos | |||
| Zorotypus longicercatus Caudell, 1927 | Jamaica | |||
| Zorotypus newi (Chao and Chen, 2000) | Taiwan | |||
| Zorotypus sechellensis Zompro, 2005 | Seychelles | |||
| Zorotypus swezeyi Caudell, 1922 | Hawaii |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kočárek, P.; Horká, I.; Kundrata, R. Molecular Phylogeny and Infraordinal Classification of Zoraptera (Insecta). Insects 2020, 11, 51. https://doi.org/10.3390/insects11010051
Kočárek P, Horká I, Kundrata R. Molecular Phylogeny and Infraordinal Classification of Zoraptera (Insecta). Insects. 2020; 11(1):51. https://doi.org/10.3390/insects11010051
Chicago/Turabian StyleKočárek, Petr, Ivona Horká, and Robin Kundrata. 2020. "Molecular Phylogeny and Infraordinal Classification of Zoraptera (Insecta)" Insects 11, no. 1: 51. https://doi.org/10.3390/insects11010051
APA StyleKočárek, P., Horká, I., & Kundrata, R. (2020). Molecular Phylogeny and Infraordinal Classification of Zoraptera (Insecta). Insects, 11(1), 51. https://doi.org/10.3390/insects11010051