Environmental Tolerance of Entomopathogenic Fungi: A New Strain of Cordyceps javanica Isolated from a Whitefly Epizootic Versus Commercial Fungal Strains
Abstract
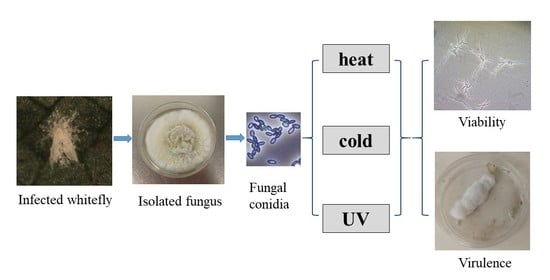
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sources and Preparation of EPF
2.2. EPF Virulence against Galleria mellonella Larvae at Environmentally Relevant Temperatures
2.3. Effect of Low and High Temperatures on Conidia Viability and Virulence
2.4. Effect of UV Light on Conidia Viability and Virulence
2.5. Data Analysis
3. Results
3.1. EPF Virulence against G. mellonella Larvae at Environmentally Relevant Temperatures
3.2. Effect of Low and High Temperatures on Conidia Viability and Virulence
3.3. Effect of UV Light on Conidia Viability and Virulence
4. Discussion
5. Conclusions
6. Patent
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brown, J.K.; Frohlich, D.R.; Rosell, R.C. The sweetpotato or silverleaf whiteflies: Biotypes of Bemisia tabaci or a species complex? Annu. Rev. Entomol. 1995, 40, 511–534. [Google Scholar] [CrossRef]
- De Barro, P.; Liu, S.-S.; Boykin, L.M.; Dinsdale, A.B. Bemisia tabaci: A statement of species status. Annu. Rev. Èntomol. 2011, 56, 1–19. [Google Scholar] [CrossRef]
- Brown, J.K.; Czosnek, H. Whitefly transmission of plant viruses. Adv. Bot. Res. 2002, 36, 65–100. [Google Scholar] [CrossRef]
- Jones, D.R. Plant viruses transmitted by whiteflies. Eur. J. Plant Pathol. 2003, 109, 195–219. [Google Scholar] [CrossRef]
- Roberts, P. Silverleaf Whitefly Deaths in Georgia Cotton Likely Fungal Related. Farm Progress, 2017. Available online: https://www.farmprogress.com/cotton/silverleaf-whitefly-deaths-georgia-cotton-likely-fungal-related (accessed on 12 September 2019).
- Kepler, R.M.; Luangsa-Ard, J.J.; Hywel-Jones, N.L.; Quandt, C.A.; Sung, G.-H.; Rehner, S.A.; Aime, M.C.; Henkel, T.W.; Sanjuan, T.; Zare, R.; et al. A phylogenetically-based nomenclature for Cordycipitaceae (Hypocreales). IMA Fungus 2017, 8, 335–353. [Google Scholar] [CrossRef] [PubMed]
- Hajek, A.E.; Meyling, N.V. Fungi. In Ecology of Invertebrate Diseases; Hajek, A.E., Shapiro-Ilan, D.I., Eds.; Wiley: Oxford, UK, 2017; pp. 327–377. [Google Scholar]
- Wraight, S.P.; Inglis, G.D.; Goettel, M.S. Fungi. In Field Manual of Techniques in Invertebrate Pathology; Springer: Dordrecht, The Netherlands, 2007; pp. 223–248. [Google Scholar]
- Wu, S.; Reddy, G.V.; Jaronski, S.T. Advances in microbial insect control in horticultural ecosystem. In Sustainable Development and Biodiversity 2; Nandawani, D., Ed.; Springer: Basel, Switzerland, 2014; pp. 223–252. [Google Scholar]
- Gao, T.; Wang, Z.; Huang, Y.; Keyhani, N.O.; Huang, Z. Lack of resistance development in Bemisia tabaci to Isaria fumosorosea after multiple generations of selection. Sci. Rep. 2017, 7, 42727. [Google Scholar] [CrossRef] [Green Version]
- Jaronski, S.T. Ecological factors in the inundative use of fungal entomopathogens. BioControl 2009, 55, 159–185. [Google Scholar] [CrossRef]
- Jackson, M.A.; Dunlap, C.A.; Jaronski, S.T. Ecological considerations in producing and formulating fungal entomopathogens for use in insect biocontrol. BioControl 2009, 55, 129–145. [Google Scholar] [CrossRef]
- Inglis, G.D.; Enkerli, J.; Goettel, M.S. Laboratory techniques used for entomopathogenic fungi. In Manual of Techniques in Invertebrate Pathology, 2nd ed.; Academic Press: San Diego, CA, USA, 2012; pp. 189–253. [Google Scholar]
- Miętkiewski, R.; Tkaczuk, C.; Żurek, M.; Van Der Geest, L.P. Temperature requirements of four entomopathogenic fungi. Acta Mycol. 2014, 29, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Shapiro-Ilan, D.I.; Cottrell, T.E.; Jackson, M.A.; Wood, B.W. Virulence of Hypocreales fungi to pecan aphids (Hemiptera: Aphididae) in the laboratory. J. Invertebr. Pathol. 2008, 99, 312–317. [Google Scholar] [CrossRef]
- Shapiro-Ilan, D.I.; Hazir, S.; Lete, L. Viability and virulence of entomopathogenic nematodes exposed to ultraviolet radiation. J. Nematol. 2015, 47, 184–189. [Google Scholar] [PubMed]
- SAS Institute Inc. SAS® 9.4 Procedures Guide; SAS Institute Inc.: Cary, NC, USA, 2016. [Google Scholar]
- Abbott, W.S. A method for computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Ekesi, S.; Maniania, N.K.; Ampong-Nyarko, K. Effect of temperature on germination, radial growth and virulence of Metarhizium anisopliae and Beauveria bassiana on Megalurothrips sjostedti. Biocontrol Sci. Technol. 1999, 9, 177–185. [Google Scholar] [CrossRef]
- Cabanillas, H.E.; Jones, W.A. Effects of temperature and culture media on vegetative growth of an entomopathogenic fungus Isaria sp. (Hypocreales: Clavicipitaceae) naturally affecting the whitefly, Bemisia tabaci in Texas. Mycopathologia 2009, 167, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, H.E.; De León, J.H.; Humber, R.A.; Murray, K.D.; Jones, W.A. Isaria poprawskii sp. nov. (Hypocreales: Cordycipitaceae), a new entomopathogenic fungus from Texas affecting sweet potato whitefly. Mycoscience 2013, 54, 158–169. [Google Scholar] [CrossRef]
- Souza, R.K.; Azevedo, R.F.; Lobo, A.O.; Rangel, D.E. Conidial water affinity is an important characteristic for thermotolerance in entomopathogenic fungi. Biocontrol Sci. Technol. 2014, 24, 448–461. [Google Scholar] [CrossRef]
- Devi, K.U.; Sridevi, V.; Mohan, C.M.; Padmavathi, J. Effect of high temperature and water stress on in vitro germination and growth in isolates of the entomopathogenic fungus Beauveria bassiana (Bals.) Vuillemin. J. Invertebr. Pathol. 2005, 88, 181–189. [Google Scholar] [CrossRef]
- Rangel, D.E.; Braga, G.U.; Anderson, A.J.; Roberts, D.W. Variability in conidial thermotolerance of Metarhizium anisopliae isolates from different geographic origins. J. Invertebr. Pathol. 2005, 88, 116–125. [Google Scholar] [CrossRef]
- Zimmermann, G. Effect of high temperatures and artificial sunlight on the viability of conidia of Metarhizium anisopliae. J. Invertebr. Pathol. 1982, 40, 36–40. [Google Scholar] [CrossRef]
- Fernandes, É.K.K.; Rangel, D.E.N.; Moraes, Á.M.; Bittencourt, V.R.E.P.; Roberts, D.W. Cold activity of Beauveria and Metarhizium, and thermotolerance of Beauveria. J. Invertebr. Pathol. 2008, 98, 69–78. [Google Scholar] [CrossRef]
- Fargues, J.; Goettel, M.S.; Smits, N.; Ouedraogo, A.; Vidal, C.; Lacey, L.A.; Lomer, C.J.; Rougier, M. Variability in susceptibility to simulated sunlight of conidia among isolates of entomopathogenic Hyphomycetes. Mycopathologia 1996, 135, 171–181. [Google Scholar] [CrossRef]
- Fernandes, É.K.K.; Rangel, D.E.N.; Moraes, Á.M.; Bittencourt, V.R.E.P.; Roberts, D.W. Variability in tolerance to UV-B radiation among Beauveria spp. isolates. J. Invertebr. Pathol. 2007, 96, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Braga, G.U.L.; Flint, S.D.; Miller, C.D.; Anderson, A.J.; Roberts, D.W. Both solar UVA and UVB radiation impair conidial culturability and delay germination in the entomopathogenic fungus Metarhizium anisopliae. Photochem. Photobiol. 2007, 74, 734–739. [Google Scholar] [CrossRef]
- Braga, G.Ú.L.; Flint, S.D.; Miller, C.D.; Anderson, A.J.; Roberts, D.W. Variability in response to UV-B among species and strains of Metarhizium isolated from sites at latitudes from 61° N to 54° S. J. Invertebr. Pathol. 2001, 78, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, É.; Da Silva, S.H.; Marques, E.D.R.; Roberts, D.W.; Braga, G.Ú.L. Quantification of cyclobutane cyrimidine dimers induced by UVB radiation in conidia of the fungi Aspergillus fumigatus, Aspergillus nidulans, Metarhizium acridum and Metarhizium robertsii. Photochem. Photobiol. 2010, 86, 1259–1266. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Toews, M.D.; Oliveira-Hofman, C.; Behle, R.W.; Simmons, A.M.; Shapiro-Ilan, D.I. Environmental Tolerance of Entomopathogenic Fungi: A New Strain of Cordyceps javanica Isolated from a Whitefly Epizootic Versus Commercial Fungal Strains. Insects 2020, 11, 711. https://doi.org/10.3390/insects11100711
Wu S, Toews MD, Oliveira-Hofman C, Behle RW, Simmons AM, Shapiro-Ilan DI. Environmental Tolerance of Entomopathogenic Fungi: A New Strain of Cordyceps javanica Isolated from a Whitefly Epizootic Versus Commercial Fungal Strains. Insects. 2020; 11(10):711. https://doi.org/10.3390/insects11100711
Chicago/Turabian StyleWu, Shaohui, Michael D. Toews, Camila Oliveira-Hofman, Robert W. Behle, Alvin M. Simmons, and David I. Shapiro-Ilan. 2020. "Environmental Tolerance of Entomopathogenic Fungi: A New Strain of Cordyceps javanica Isolated from a Whitefly Epizootic Versus Commercial Fungal Strains" Insects 11, no. 10: 711. https://doi.org/10.3390/insects11100711
APA StyleWu, S., Toews, M. D., Oliveira-Hofman, C., Behle, R. W., Simmons, A. M., & Shapiro-Ilan, D. I. (2020). Environmental Tolerance of Entomopathogenic Fungi: A New Strain of Cordyceps javanica Isolated from a Whitefly Epizootic Versus Commercial Fungal Strains. Insects, 11(10), 711. https://doi.org/10.3390/insects11100711