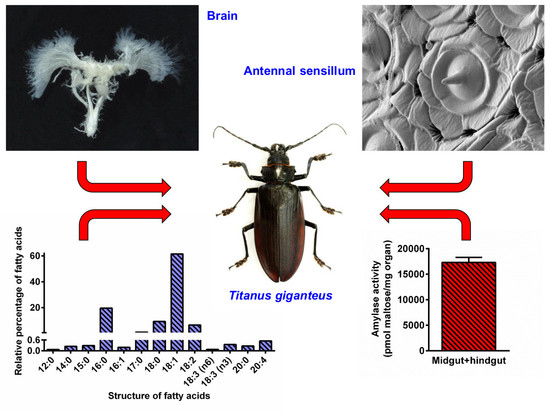
First Comprehensive Study of a Giant among the Insects, Titanus giganteus: Basic Facts from Its Biochemistry, Physiology, and Anatomy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Titanus Origin
2.2. Dissection
2.3. Mallory Staining and Microscopy of Testes
2.4. Scanning Electron Microscopy—Sample Preparation
2.5. Digestive Enzyme Activity Determinations
2.6. Mass Spectrometry Determination of Muscle Lipids
2.7. Data Presentation
3. Results
3.1. Brief Summary of External Morphology
3.2. Analyses of Selected Sensory Organs
3.3. Internal Organs and Tissues
3.4. Biochemical Analyses
4. Discussion
4.1. Structure of External Titanus Sensilla and Their Possible Functions
4.2. Titanus Central Nervous System (CNS) and Compound Eyes
4.3. Titanus Gut and Fat Body
4.4. Titanus Muscles and Their Lipid Content
4.5. Titanus Testes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Raye de Breukelerwaert, J. Catalogue du cabinet célèbre et très renommé d’objets d’Histoire Naturelle delaissé par le très noble sieur Joan Raye seigneur de Breukelerwaert à Amsterdam; Van Cleef: Amsterdam, 1827; pp. 68–71. [Google Scholar]
- Lameere, A.A.L. Révision des Prionides. Dixième mémoire. Titanines Ann. de la société Entomol. de Belg. Brux. 1904, 48, 309–352. [Google Scholar]
- Le Moult, E. Captures et Biologie. Bull. Soc. Entomol. Fr. 1909, 55–56. [Google Scholar]
- Bleuzen, P. Les coleopteres du monde: Prioninae 1. Sci. Nat. Venette 1994, 21, 1–92. [Google Scholar]
- Tavakilian, G. Base de données Titan sur les cerambycidés ou longicornes. 2018. Available online: http://titan.gbif.fr/index.html (accessed on 12 May 2019).
- Monné, M.A. Catalogue of the Cerambycidae (Coleoptera) of the neotropical region. Part II. Subfamilies Lepturinae, Necydalinae, Parandrinae, Prioninae, Spondylidinae and families Oxypeltidae, Vesperidae, and Disteniidae. 2018. Available online: http://cerambyxcat.com/Parte3_Prioninae_Lepturinae_2018.pdf (accessed on 12 May 2019).
- Lacordaire, J.T. Notice sur l’entomologie de la Guyane française. Ann. de la société Entomol. de Fr. Paris 1832, 1, 348–366. [Google Scholar]
- Drury, D.; Westwood, J.O. Illustrations of Exotic Entomology, Containing Upwards of Six Hundred and Fifty Figures and Descriptions of Foreign Insects Interspersed with Remarks and Reflections on Their Nature and Properties, 1st ed.; Henry, G., Ed.; Bohn: London, UK, 1836. [Google Scholar]
- Sturm, J. Catalog der Käfer-Sammlung von Jacob Sturm; Gedruckt auf Kosten des Verfassers: Nürnberg, Germany, 1843. [Google Scholar]
- Thomson, J. Systema Cerambycidarum ou exposé de tous les genres compris dans la famille des Cérambycides et familles limitrophes. Mémoires de la Société R. des Sci. de Liège 1864, 19, 1–540. [Google Scholar]
- Bates, H.W., IX. Contributions to an Insect Fauna of the Amazon Valley (Coleoptera, Prionides). Trans. Entomol. Soc. Lond. 1869, I, 37–58. [Google Scholar] [CrossRef]
- Schloss, P.D.; Delalibera, I.; Handelsman, J.; Raffa, K.F. Bacteria associated with the guts of two woodboring beetles: Anoplophora glabripennis and Saperda vestita (Cerambycidae). Environ. Entomol. 2006, 35, 625–629. [Google Scholar] [CrossRef]
- Mohammed, W.S.; Ziganshina, E.E.; Shagimardanova, E.I.; Gogoleva, N.E.; Ziganshin, A.M. Comparison of intestinal bacterial and fungal communities across various xylophagous beetle larvae (Coleoptera: Cerambycidae). Sci. Rep. 2018, 8, 10073. [Google Scholar] [CrossRef]
- Weber, M.; Darzens, D.; Coulombel, C.; Foglietti, M.J.; Charas, C. Purification and some properties of two amylases from Phoracantha semipunctata larvae. Comp. Biochem. Physiol. B 1985, 80, 57–60. [Google Scholar] [CrossRef]
- Patil, N.K.; Raut, G.A.; Gaikwand, S.M. Activity of midgut amylase in Aeolesthes holosericea Fabricius (Coleoptera: Cerambycidae). J. Entomol. Zool. Stud. 2016, 4, 5–48. [Google Scholar]
- Scrivener, A.M.; Watanabe, H.; Noda, H. Diet and carbohydrate digestion in the yellowspotted longicorn beetle Psacothea hilaris. J. Insect Physiol. 1997, 43, 1039–1052. [Google Scholar] [CrossRef]
- Bian, X.; Shaw, B.D.; Han, Y.; Christeller, J.T. Midgut proteinase activities in larvae of Anoplophora glabripennis (Coleoptera: Cerambycidae) and their interaction with proteinase inhibitors. Arch. Insect Biochem. Physiol. 1996, 31, 23–37. [Google Scholar] [CrossRef]
- Johnson, K.S.; Rabosky, D. Phylogenetic distribution of cysteine proteinases in beetles: Evidence for an evolutionary shift to an alkaline digestive strategy in Cerambycidae. Comp. Biochem. Physiol. B 2000, 126, 609–619. [Google Scholar] [CrossRef]
- Torres-Castillo, J.A.; Aguirre-Mancilla, C.L.; Gutierréz-Diéz, A.; Sinagawa-García, S.R.; Torres-Acosta, R.I.; García-Zambrano, E.A.; Aguirre-Arzola, V.; Zavala-García, F. Intestinal proteases of Moneilema armatum (Coleoptera: Cerambycidae) fed with Opunthia cladodes. Rev. Colomb. Entomol. 2015, 41, 249–256. [Google Scholar]
- Kukor, J.J.; Martin, M.M. Cellulose digestion in Monochamus marmorator Kby. (Coleoptera: Cerambycidae): Role of acquired fungal enzymes. J. Chem. Ecol. 1986, 12, 1057–1070. [Google Scholar] [CrossRef] [Green Version]
- Park, D.S.; Oh, H.W.; Jeong, W.J.; Kim, H.; Park, H.Y.; Bae, K.S. A culture-based study of the bacterial communities within the guts on nine longicorn beetle species and their exo-enzyme producing properties for degrading xylan and pectin. J. Microbiol. 2007, 45, 394–401. [Google Scholar]
- Chang, C.J.; Wu, C.P.; Lu, S.C.; Chao, A.L.; Ho, T.H.D.; Yu, S.M.; Chao, Y.C. A novel exo-cellulase from white spottted longhorn beetle (Anoplophora malasiaca). Insect Biochem. Mol. Biol. 2012, 42, 629–636. [Google Scholar] [CrossRef]
- Pauchet, Y.; Kirsch, R.; Giraud, S.; Vogel, H.; Heckel, D.G. Identification and characterization of plant cell wall degrading enzymes from three glycoside hydrolase families in the cerambycid beetle Apriona japonica. Insect Biochem. Mol. Biol. 2014, 49, 1–13. [Google Scholar] [CrossRef]
- Silva, I. Morfologia do tubo digestivo da larva de Oncideres saga saga (Dalman, 1823) (Coleoptera, Cerambycidae). Acta Biol. Par. 1975, 4, 227–239. [Google Scholar] [CrossRef] [Green Version]
- Silva, I.; Souza, V.B.V. Ultrastructural aspects of the anterior mid gut of Oncideres saga saga (Dalman, 1823)—larva (Coleoptera, Cerambycidae). Rev. Bras. Entomol. S. P. 1981, 25, 103–112. [Google Scholar]
- Haack, R.A. Feeding biology of Cerambycids. In Cerambycidae of the World; Biology and Pest Management; Wang, O., Ed.; CRC Press Boca: Boca Raton, FL, USA, 2017; pp. 105–124. [Google Scholar]
- Yi, D.A.; Kuprin, A.V.; Lee, Y.H.; Bae, Y.J. Newly developed fungal diet for artificial rearing of the endangered long-horned beetle Callipogon relictus (Coleoptera: Cerambycidae). Entomol. Res. 2017, 47, 373–379. [Google Scholar] [CrossRef]
- Lee, S.G.; Kim, C.; Choi, I.J.; Kuprin, A.V.; Lim, J. A review of host plants of Callipogon (Eoxenus) relictus Semenov (Coleoptera: Cerambycidae: Prioninae), a Korea natural monument, with a new host, Quercus aliena Blume. J. Asia-Pac. Entomol. 2010, 22, 353–358. [Google Scholar] [CrossRef]
- Edwards, J.S. Observation on the ecology and behaviour of the Huhu beetle, Prionoplus reticularis White (Col. Ceramb.). Trans. R. Soc. N. Z. 1961, 88, 727–731. [Google Scholar]
- Edwards, J.S. On the reproduction of Prionoplus reticularis (Coleoptera: Cerambycidae), with general remarks on reproduction in the Cerambycidae. J. Cell. Sci. 1961, 102, 519–522. [Google Scholar]
- Mansour, K.; Mansour-Bek, J.J. On the digestion of wood by insects. J. Exp. Biol. 1933, 11, 243–256. [Google Scholar]
- Benham, G.S. Gross morphology and transformation of the digestive tract of Prionus laticollis (Coleoptera: Cerambycidae). Ann. Entomol. Soc. Am. 1970, 63, 1413–1419. [Google Scholar] [CrossRef]
- Benham, G.S.R.; Farrar, J. Notes on the biology of Prionus laticollis (Coleoptera: Cerambycidae). Can. Entomol. 1976, 108, 569–576. [Google Scholar] [CrossRef]
- Rodstein, J.; McElfresh, J.S.; Barbour, J.D.; Ray, A.M.; Hanks, L.M.; Millar, J.G. Identification and synthesis of a female-produced sex pheromone for the cerambycid beetle Prionus californicus. J. Chem. Ecol. 2009, 35, 590–600. [Google Scholar] [CrossRef]
- Kaiser, A.; Klok, J.; Socha, J.J.; Lee, W.K.; Quinlan, M.C.; Harrison, J.F. Increase in tracheal investment with beetle size supports hypothesis of oxygen limitation on insect gigantism. Proc. Natl. Acad. Sci. USA 2007, 104, 13198–13203. [Google Scholar] [CrossRef] [Green Version]
- Levine, J.D.; Sauman, I.; Imbalzano, M.; Reppert, S.M.; Jackson, F.R. Period protein from the giant silkmoth Antheraea pernyi functions as a circadian clock element in Drosophila melanogaster. Neuron 1995, 15, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Bernfeld, P. Amylases, α and β. In Methods in Enzymology; Colowick, S.P., Kaplan, N.O., Eds.; Academic Press: New York, NY, USA, 1955; Volume 1, pp. 49–58. [Google Scholar]
- Kodrík, D.; Vinokurov, K.; Tomčala, A.; Socha, R. The effect of adipokinetic hormone on midgut characteristics in Pyrrhocoris apterus L. (Heteroptera). J. Insect Physiol. 2012, 58, 194–204. [Google Scholar] [CrossRef]
- Frugoni, J.A.C. Tampone universale di Britton e Robinson a forza ionica costante. Gazz. Chim. Ital. 1957, 87, 403–407. [Google Scholar]
- Elpidina, E.N.; Vinokurov, K.S.; Gromenko, V.A.; Rudenskaya, Y.A.; Dunaevsky, Y.E.; Zhuzhikov, D.P. Compartmentalization of proteinases and amylases in Nauphoeta cinerea midgut. Arch. Insect Biochem. Physiol. 2012, 48, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Roberts, I.M. Hydrolysis of 4-methylumbelliferyl butyrate: A convenient and sensitive fluorescent assay for lipase activity. Lipids 1985, 20, 243–247. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Tomčala., A.; Kyselová, V.; Schneedorferová, I.; Opekarová, I.; Moos, M.; Urajová, P.; Kručinská, J.; Oborník, M. Separation and identification of lipids in the photosynthetic cousins of Apicomplexa Chromera velia and Vitrella brassicaformis. J. Sep. Sci. 2017, 40, 3402–3413. [Google Scholar]
- Appelqvist, L.A. Rapid methods of lipid extraction and fatty acid methyl ester preparation for seed and leaf tissue with special remarks on preventing accumulation of lipid contaminants. Chem. Sci. 1968, 28, 551–570. [Google Scholar]
- Williams, D.M. Chapter 30: Largest. In Book of Insect Records; University of Florida: Gainesville, FL, USA, 2001; Available online: http://entnemdept.ufl.edu/walker/ufbir/chapters/chapter_30.shtml (accessed on 21 June 2019).
- Monné, M.A. Catalogue of the Cerambycidae (Coleoptera) of the neotropical region. Part III. Subfamilies Parandrinae, Prioninae, Anoplodermatinae, Aseminae, Spondylidinae, Lepturinae, Oxypeltinae, and addenda to the Cerambycinae and Lamiinae. Zootaxa 2006, 1212, 1–244. [Google Scholar] [CrossRef]
- Shanbhag, S.R.; Müller, B.; Steibrecht, R.A. Atlas of olfactory organs of Drosophila melanogaster. 1. Types, external organization, innervation and distribution of olfactory sensilla. Int. J. Insect Morphol. Embryol. 1999, 28, 377–397. [Google Scholar] [CrossRef]
- Altner, H.; Prillinger, L. Ultrastructure of invertebrate chemo-, thermo-, and hygroreceptors and its functional significance. Int. Rev. Cytol. 1980, 67, 69–139. [Google Scholar]
- Altner, H.; Routil, C.; Loftus, R. The structure of bimodal chemo-, thermo-, and hygroreceptive sensilla on the antenna of Locusta migratoria. Cell Tissue Res. 1981, 215, 289–308. [Google Scholar] [CrossRef] [PubMed]
- Hunger, T.; Steinbrecht, R.A. Functional morphology of a double-walled multiporous olfactory sensillum: The sensillum coeloconicum of Bombyx mori (Insecta, Lepidoptera). Tissue Cell 1998, 30, 14–29. [Google Scholar] [CrossRef]
- Steinbrecht, R.A. Olfactory receptors. In Atlas of Arthropod Sensory Receptors-Dynamic Morphology in Relation to Function; Eguchi, E., Tominaga, Y., Eds.; Springer Verlag: Tokyo, Japan, 1999; pp. 155–176. [Google Scholar]
- Di Palma, A.; Pistillo, M.; Griffo, R.; Garonna, A.P.; Germinara, G.S. Scanning electron microscopy of the antennal sensilla and their secretion analysis in adults of Aromia bungii (Faldermann, 1835) (Coleoptera, Cerambycidae). Insects 2019, 10, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, E.S.; Römer, H. Sensory structures on the antennal flagella of two katydid species of the genus Mecopoda (Orthoptera, Tettigonidae). Micron 2016, 90, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Weyda, F.; Štys, P. Coxal setal organs of Machilidae and their homologues on the genitalia. Acta Entomol. Bohemoslov. 1974, 71, 51–52. [Google Scholar]
- Eilers, E.J.; Talarico, G.; Hansson, B.; Hilker, M.; Reinecke, A. Sensing the underground – ultrastructure and function of sensory organs in root-feeding Melolontha melolontha (Coleoptera: Scarabaeinae) larvae. PLoS ONE 2012, 7, e41357. [Google Scholar] [CrossRef]
- Voigt, D.; Takanashi, T.; Tsuchihara, K.; Yazaki, K.; Kuroda, K.; Tsubaki, R.; Hosoda, N. Strongest grip on the rod: Tarsal morphology and attachment of Japanese pine sawyer beetles. Zool. Lett. 2017, 3, 16. [Google Scholar] [CrossRef] [Green Version]
- Gorb, E.V.; Hosoda, N.; Miksch, C.; Gorb, S.N. Slippery pores: Anti-adhesive effect of nanoporous substrates on the beetle attachment system. J. R. Soc. Interface 2019, 7, 1571–1579. [Google Scholar] [CrossRef] [Green Version]
- Bullock, J.M.R.; Federle, W. Division of labour and sex differences between fibrillar, tarsal adhesive pads in beetles: Effective elastic modulus and attachment performance. J. Exp. Biol. 2009, 212, 1876–1888. [Google Scholar] [CrossRef] [Green Version]
- Niederegger, S.; Gorb, S.; Jiao, Y.K. Contact behaviour of tenent setae in attachment pads of the blowfly Calliphora vicina (Diptera, Calliphoridae). J. Comp. Physiol. A 2002, 187, 961–970. [Google Scholar] [CrossRef]
- Büscher, T.H.; Buckley, T.R.; Grohmann, C.; Gorb, S.N.; Bradler, S. The evolution of tarsal adhesive microstructures in stick and leaf insects (Phasmatodea). Front. Ecol. Evol. 2018, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Chapman, R.F. The Insects, Structure and Function, 4th ed.; Cambridge University Press: Cambridge, UK, 1998; pp. 1–770. [Google Scholar]
- Sehadová, H.; Šauman, I.; Sehnal, F. Immunocytochemical distribution of pigment dispersing hormone in the cephalic ganglia of polyneopteran insects. Cell Tissue Res. 2003, 312, 113–125. [Google Scholar] [CrossRef]
- Mitchell, R.F.; Hall, L.P.; Reagel, P.F.; McKenna, D.D.; Baker, T.C.; Hildebrand, J.G. Odorant receptors and antennal lobe morphology offer a new approach to understanding the olfactory biology of the Asian longhorned beetle. J. Comp. Physiol. A 2017, 203, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Gokan, N.; Hosobuchi, Y. Fine structure of the compound eyes of longicorn beetles (Coleoptera: Cerambycidae). Appl. Entomol. Zool. 1979, 14, 12–27. [Google Scholar] [CrossRef]
- Wachmann, E. Untersuchungen zur Feinstruktur der Augen von Bockka fern (Coleoptera, Cerambycidae). Zoomorphology 1979, 92, 19–48. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B.; Mishra, M. A six-rhabdomere, open rhabdom arrangement in the eye of the chrysanthemum beetle Phytoecia rufiventris: Some ecophysiological predictions based on eye anatomy. Biocell 2009, 33, 115–120. [Google Scholar]
- Crowson, R.A. The Biology of the Coleoptera; Academic Press: New York, NY, USA, 1981; pp. 1–802. [Google Scholar]
- Caveney, S. The phylogenetic significance of ommatidium structure in the compound eye of polyphagan beetles. Can. J. Zool. 1986, 64, 1787–1819. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B. The dioptric system of beetle compound eyes. In The Compound Eye and Vision of Insects; Horridge, G.A., Ed.; Clarendon Press: Oxford, UK, 1975; pp. 299–313. [Google Scholar]
- Mishra, M.; Meyer-Rochow, V.B. Fine structure of the compound eye of the fungus beetle Neotriplax lewisi (Coleoptera, Cucujiformia, Erotylidae). Invertebr. Biol. 2006, 125, 265–278. [Google Scholar] [CrossRef]
- Jia, L.P.; Liang, A.P. An apposition-like compound eye with a layered rhabdom in the small diving beetle Agabus japonicus (Coleoptera, Dytiscidae). J. Morphol. 2014, 275, 1273–1283. [Google Scholar] [CrossRef]
- Niven, J.E.; Graham, C.M.; Burrows, M. Diversity and evolution of insect nervous cord. Annu. Rev. Entomol. 2008, 53, 253–271. [Google Scholar] [CrossRef]
- Crowson, R.A. The phylogeny of Coleoptera. Annu. Rev. Entomol. 1960, 5, 111–134. [Google Scholar] [CrossRef]
- Calder, A.A. The alimentary canal and nervous system of Curculionidae (Coleoptera): Gross morphology and systematic significance. J. Nat. Hist. 1989, 23, 1205–1265. [Google Scholar] [CrossRef]
- Mohammadi, H.; Venkataraman Ramamurthy, V.; Subrahmanyam, B. Configuration of nerve cord and characterization of brain neurosecretory cells in adult firefly, Luciola gorhami (Coleoptera: Lampyridae). J. Crop. Prot. 2016, 5, 179–187. [Google Scholar] [CrossRef] [Green Version]
- Penteado-Dias, A.M. Comparative study of the neural cord in the – Cerambycidae (Coleoptera). Rev. Bras. Entomol. 1984, 28, 223–243. [Google Scholar]
- Snodgrass, R.E. Principles of Insect Morphology; McGraw-Hill: New York, NY, USA, 1935; pp. 1–667. [Google Scholar]
- Martin, M.M. Invertebrate-Microbial Interactions. Ingested Fungal Enzymes in Arthropod Biology; Cornell University Press: Ithaca and London, UK, 1987; pp. 1–148. [Google Scholar]
- Crook, D.J.; Prabhakar, S.; Oppert, B. Protein digestion in larvae of the red oak borer Enaphalodes rufulus. Physiol. Entomol. 2009, 34, 152–157. [Google Scholar] [CrossRef] [Green Version]
- Sharifi, M.; Chitgar, M.G.; Ghadamyari, M.; Ajamhasani, M. Identification and characterization of midgut digestive proteases from the rosaceous branch borer, Osphranteria coerulescens Redtenbacher (Coleoptera: Cerambycidae). Rom. J. Biochem. 2012, 49, 33–47. [Google Scholar]
- Zibaee, A. Digestive proteolytic profile in Stromatium fulvum Villers (Coleoptera: Cerambycidae). Rom. J. Biochem. 2014, 51, 17–30. [Google Scholar]
- Canavoso, L.E.; Jouni, Z.E.; Karnas, K.J.; Pennington, J.E.; Wells, M.A. Fat metabolism in insects. Annu. Rev. Nutr. 2001, 21, 23–46. [Google Scholar] [CrossRef]
- Howard, R.W.; Stanley-Samuelson, D.W. Fatty acid composition of fat body and Malpighian tubules of the tenebrionid beetle, Zophobas atratus: Significance in eicosanoid-mediated physiology. Comp. Biochem. Physiol. B 1996, 115, 429–437. [Google Scholar] [CrossRef]
- Tomčala, A.; Bártů, I.; Šimek, P.; Kodrík, D. Locust adipokinetic hormone mobilizes diacylglycerols selectively. Comp. Biochem. Physiol. B 2010, 156, 26–32. [Google Scholar] [CrossRef]
- Canavoso, L.E.; Frede, S.; Rubiolo, E.R. Metabolic pathways for dietary lipids in the midgut of hematophagous Panstrongylus megistus (Hemiptera: Reduviidae). Insect. Biochem. Mol. Biol. 2004, 34, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Stanley-Samuelson, D.W.; Jurenka, R.A.; Cripps, C.; Blomquist, G.J.; de Renobales, M. Fatty acids in insects: Composition, metabolism, and biological significance. Arch. Insect Biochem. Physiol. 1988, 9, 1–33. [Google Scholar] [CrossRef]
- Diefenbach, L.M.G.; Redaelli, L.R.; Gassen, D.N. Characterization of the internal reproductive organs and their state as diapause indicator in Phytalus sanctipauli Blanchard, 1850 (Coleoptera, Scarabaeidae). Rev. Brasil. Biol. 1998, 58, 541–546. [Google Scholar] [CrossRef]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dvořáček, J.; Sehadová, H.; Weyda, F.; Tomčala, A.; Hejníková, M.; Kodrík, D. First Comprehensive Study of a Giant among the Insects, Titanus giganteus: Basic Facts from Its Biochemistry, Physiology, and Anatomy. Insects 2020, 11, 120. https://doi.org/10.3390/insects11020120
Dvořáček J, Sehadová H, Weyda F, Tomčala A, Hejníková M, Kodrík D. First Comprehensive Study of a Giant among the Insects, Titanus giganteus: Basic Facts from Its Biochemistry, Physiology, and Anatomy. Insects. 2020; 11(2):120. https://doi.org/10.3390/insects11020120
Chicago/Turabian StyleDvořáček, Jiří, Hana Sehadová, František Weyda, Aleš Tomčala, Markéta Hejníková, and Dalibor Kodrík. 2020. "First Comprehensive Study of a Giant among the Insects, Titanus giganteus: Basic Facts from Its Biochemistry, Physiology, and Anatomy" Insects 11, no. 2: 120. https://doi.org/10.3390/insects11020120
APA StyleDvořáček, J., Sehadová, H., Weyda, F., Tomčala, A., Hejníková, M., & Kodrík, D. (2020). First Comprehensive Study of a Giant among the Insects, Titanus giganteus: Basic Facts from Its Biochemistry, Physiology, and Anatomy. Insects, 11(2), 120. https://doi.org/10.3390/insects11020120