Surviving the Antarctic Winter—Life Stage Cold Tolerance and Ice Entrapment Survival in The Invasive Chironomid Midge Eretmoptera murphyi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Sample Collection
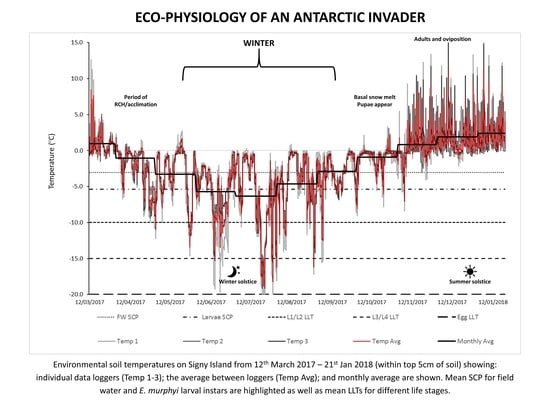
2.2. Overwintering Environmental Data
2.3. Cold-Tolerance Ability of E. Murphyi
2.3.1. Measurement of Supercooling Points
2.3.2. Cold-Tolerance Strategy of Juvenile Life Stages
2.3.3. Lower Thermal Activity Thresholds of Adults
2.4. Overwintering in Ice and Prolonged Submergence in Water
2.4.1. Field vs. Laboratory Water
2.4.2. Long-Term Ice Entrapment
2.5. Statistical Analysis
3. Results
3.1. Supercooling and Activity Thresholds
3.2. Cold-Tolerance Strategy of Different Life Stages
3.3. Overwintering in Ice and Prolonged Submergence
3.3.1. Field vs. Laboratory Water Freezing Point
3.3.2. Prolonged Ice Entrapment and Submergence
3.4. Overwintering Environmental Data
4. Discussion
Implications for E. Murphyi as an Invasive Species
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Convey, P.; Coulson, S.J.; Worland, M.R.; Sjöblom, A. The importance of understanding annual and shorter-term temperature patterns and variation in the surface levels of polar soils for terrestrial biota. Polar Biol. 2018, 41, 1587–1605. [Google Scholar] [CrossRef] [Green Version]
- Mazur, P. The role of intracellular freezing in the death of cells cooled at supraoptimal rates. Cryobiology 1977, 14, 251–272. [Google Scholar] [CrossRef]
- Bale, J.S. Insects and low temperatures: From molecular biology to distributions and abundance. Phil. Trans. Roy. Soc. B. 2002, 357, 849–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sømme, L.; Block, W. Cold hardiness of Collembola at Signy Island, maritime Antarctic. Oikos 1982, 38, 168–176. [Google Scholar] [CrossRef]
- Hazell, S.P.; Bale, J.S. Low temperature thresholds: Are chill coma and CT (min) synonymous? J. Insect Physiol. 2011, 57, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Frouz, J.; Matena, J.; Ali, A. Survival strategies of chironomids (Diptera: Chironomidae) living in temporary habitats: A review. Eur. J. Entomol. 2003, 100, 459–465. [Google Scholar] [CrossRef] [Green Version]
- Hayward, S.A.L.; Bale, J.S.; Worland, M.R.; Convey, P.; Bale, J.S. Temperature preferences of the mite, Alaskozetes antarcticus, and the collembolan, Cryptopygus antarcticus from the maritime Antarctic. Physiol. Entomol. 2003, 28, 114–121. [Google Scholar] [CrossRef]
- Davey, M.C.; Pickup, J.; Block, W. Temperature variation and its biological significance in fellfield habitats on a maritime Antarctic island. Antarct. Sci. 1992, 4, 383–388. [Google Scholar] [CrossRef]
- Bale, J.S. Classes of insect cold hardiness. Funct. Ecol. 1993, 7, 751–753. [Google Scholar]
- Bale, J.S. Insect cold hardiness: A matter of life and death. Eur. J. Entomol. 1996, 93, 369–382. [Google Scholar]
- Ditrich, T. Supercooling point is an individually fixed metric of cold tolerance in Pyrrhocoris apterus. J. Thermal Biol. 2018, 74, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Everatt, M.J.; Worland, M.R.; Bale, J.S.; Convey, P.; Hayward, S.A.L. Responses of invertebrates to temperature and water stress: A polar perspective. J. Thermal Biol. 2015, 54, 118–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Worland, M.R. Eretmoptera murphyi: Pre-adapted to survive a colder climate. Physiol. Entomol. 2010, 35, 140–145. [Google Scholar] [CrossRef]
- Block, W.; Burn, A.J.; Richard, K.J. An insect introduction to the maritime Antarctic. Biol. J. Linn. Soc. 1984, 23, 33–39. [Google Scholar] [CrossRef]
- Allegruci, G.; Carchini, G.; Todisco, V.; Convey, P.; Sbordoni, V. A molecular phylogeny of Antarctic Chironomidae and its implications for biogeographical history. Polar Biol. 2006, 29, 320–326. [Google Scholar] [CrossRef]
- Allegruci, G.; Carchini, G.; Convey, P.; Sbordoni, V. Evolutionary geographic relationships among orthocladine chironomid midges from maritime Antarctic and sub-Antarctic islands. Biol. J. Linn. Soc. 2012, 106, 258–274. [Google Scholar] [CrossRef] [Green Version]
- Bartlett, J.C.; Convey, P.; Hayward, S.A.L. Not so free range: Oviposition microhabitat and egg clustering effects Eretmoptera murphyi (Diptera Chironomidae) reproductive success. Polar Biol. 2018, 42, 271–284. [Google Scholar] [CrossRef] [Green Version]
- Everatt, M.J.; Worland, M.R.; Bale, J.S.; Convey, P.; Hayward, S.A.L. Contrasting strategies of resistance vs. tolerance to desiccation in two polar dipterans. Polar Res. 2014, 33, 22963. [Google Scholar] [CrossRef] [Green Version]
- Everatt, M.J.; Worland, M.R.; Bale, J.S.; Convey, P.; Hayward, S.A.L. Pre-adapted to the maritime Antarctic? - Rapid cold hardening of the midge, Eretmoptera murphyi. J. Insect Physiol. 2012, 58, 1104–1111. [Google Scholar] [CrossRef]
- Hodkinson, I.D.; Bird, J.M. Anoxia tolerance in high Arctic terrestrial microarthropods. Ecol. Entomol. 2004, 29, 506–509. [Google Scholar] [CrossRef]
- Everatt, M.J.; Convey, P.; Mirbahai, L.; Worland, M.R.; Bale, J.S.; Hayward, S.A.L. Can the Antarctic terrestrial midge, Eretmoptera murphyi, tolerate life in water? Ecol. Entomol. 2014, 39, 732–735. [Google Scholar] [CrossRef]
- Convey, P.; Block, W. Antarctic Diptera: Ecology, physiology and distribution. Eur. J. Entomol. 1996, 93, 1–13. [Google Scholar]
- Walton, D.W.H. The Signy Island terrestrial reference sites: XV. Micro-climate monitoring, 1972–1974. Br. Antarct. Surv. Bull. 1982, 55, 111–126. [Google Scholar]
- Benoit, J.B.; Lopez-Martinez, G.; Michaud, M.R.; Elnitsky, M.A.; Lee, R.E.; Denlinger, D.L. Mechanisms to reduce dehydration stress in larvae of the Antarctic midge, Belgica antarctica. J. Insect Physiol. 2007, 53, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Elnitsky, M.A.; Hayward, S.A.L.; Rinehart, J.P.; Denlinger, D.L.; Lee, R.E. Cryoprotective dehydration and the resistance to inoculative freezing in the Antarctic midge, Belgica antarctica. J. Exp. Biol. 2008, 211, 524–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayward, S.A.L.; Rinehart, J.P.; Sandro, L.H.; Lee, R.; Denlinger, D. Slow dehydration promotes desiccation and freeze tolerance in the Antarctic midge Belgica antarctica. J. Exp. Biol. 2007, 210, 836–844. [Google Scholar] [CrossRef] [Green Version]
- Bartlett, J.C.; Convey, P.; Hayward, S.A.L. Life cycle and phenology of an Antarctic invader – the flightless chironomid midge, Eretmoptera murphyi. Polar Biol. 2018, 42, 115–130. [Google Scholar] [CrossRef] [Green Version]
- Convey, P. Aspects of the biology of the midge, Eretmoptera murphyi Schaeffer (Diptera, Chironomidae), introduced to Signy island, maritime Antarctic. Polar Biol. 1992, 12, 653–657. [Google Scholar] [CrossRef]
- White, N.; Bale, J.S.; Hayward, S.A.L. Life-history changes in the cold tolerance of the two-spot spider mite: Applications in pest control and establishment risk assessment. Physiol. Entomol. 2018, 43, 334–345. [Google Scholar] [CrossRef] [Green Version]
- Danks, H.V. Overwintering of some north temperate and Arctic Chironomidae. Can. Entomol. 1971, 103, 1875–1910. [Google Scholar] [CrossRef]
- Lencioni, V. Survival strategies of freshwater insects in cold environments. J. Limnol. 2004, 63, 45–55. [Google Scholar] [CrossRef]
- Scholander, P.F.; Flagg, W.; Hock, R.J.; Irving, L. Studies on the physiology of frozen plants and animals in the arctic. J. Cell. Comp. Physiol. 1953, 41, 1–56. [Google Scholar] [CrossRef]
- Tokeshi, M. Life cycles and population dynamics. In The Chironomidae: Biology and Ecology of Non-Biting Midges; Armitage, P.D., Cranston, P.S., Pinder, L.C.V., Eds.; Chapman & Hall: London, UK, 1995; pp. 225–268. [Google Scholar]
- Bouchard, R.W.; Carillo, M.A.; Kells, S.; Ferrington, L.C. Freeze tolerance in larvae of the winter-active Diamesa mendotae Muttkowski (Diptera: Chironomidae): A contrast to adult strategy for survival at low temperatures. Hydrobiologia 2006, 568, 403–416. [Google Scholar] [CrossRef]
- Lee, R.E.; Denlinger, D. Cold tolerance in diapausing and non-diapausing stages of the flesh fly, Sarcophaga crassipalpis. Physiol. Entomol. 2008, 10, 309–315. [Google Scholar] [CrossRef]
- Burgi, L.; Mills, N.J. Cold tolerance of the overwintering larval instars of light brown apple moth Epiphyas postvittana. J. Insect Physiol. 2010, 56, 1645–1650. [Google Scholar] [CrossRef]
- Swain, J.A.; Judd, G.J.R.; Cory, J.S. Cold tolerance of the spring-feeding larvae of the eyespotted bud moth, Spilonota ocellana (Lepidoptera: Tortricidae). Can. Entomol. 2017, 149, 291–299. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Synthesis Report. International Panel on Climate Change. Available online: https://www.ipcc.ch/report/ar5/syr/ (accessed on 24 January 2019).
- Walther, G.; Post, E.; Convey, P.; Menzel, A.; Parmesank, C.; Beebee, T.J.C.; Fromentin, J.I.; Ove, H.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef]
- Lehmann, P.; Kaunisto, S.; Kostal, V.; Margus, A.; Zahradnickova, H.; Lindstrom, L. Comparative ecophysiology of cold tolerance related traits: Assessing range expansion potential for an invasive insect at high latitude. Physiol. Biochem. Zool. 2015, 88, 254–265. [Google Scholar] [CrossRef]
- Pertierra, L.R.; Aragón, P.; Shaw, J.D.; Bergstrom, D.M.; Terauds, A.; Olalla-Tárraga, M.A. Global thermal niche models of two European grasses show high invasion risks in Antarctica. Global Change Biol. 2016, 23, 2863–2873. [Google Scholar] [CrossRef] [Green Version]
- Chown, S.L.; Gaston, K.J. Exploring links between physiology and ecology at macro-scales: The role of respiratory metabolism in insects. Biol. Rev. 1999, 74, 87–120. [Google Scholar] [CrossRef]
- Convey, P. The influence of environmental characteristics on life history attributes of Antarctic terrestrial biota. Biol. Rev. 1996, 71, 191–225. [Google Scholar] [CrossRef]
- Bale, J.S.; Hayward, S.A.L. Insect overwintering in a changing climate. J. Exp. Biol. 2010, 213, 980–994. [Google Scholar] [CrossRef] [Green Version]
- Sømme, L. Supercooling and winter survival in terrestrial arthropods. Comp. Biochem. Physiol. 1982, 73, 519–543. [Google Scholar] [CrossRef]
- Worland, M.R.; Convey, P. The significance of the moult cycle to cold tolerance in the Antarctic collembolan Cryptopygus antarcticus. J. Insect Physiol. 2008, 54, 1281–1285. [Google Scholar] [CrossRef] [PubMed]
- Rezendez, E.L.; Castañeda, L.; Santos, M. Tolerance landscapes in thermal ecology. Funct. Ecol. 2014, 28, 799–809. [Google Scholar] [CrossRef]
- Terblanche, J.S.; Hoffmann, A.A.; Mitchell, K.A.; Rako, L.; Le Roux, P.C.; Chown, S.L. Ecologically relevant measures of tolerance to potentially lethal temperatures. J. Exp. Biol. 2011, 214, 3713–3725. [Google Scholar] [CrossRef] [Green Version]
- Everatt, M.; Convey, P.; Worland, M.; Bale, J.; Hayward, S.A.L. Are the Antarctic dipteran, Eretmoptera murphyi, and Arctic collembolan, Megaphorura arctica, vulnerable to rising temperatures? Bull. Entomol. Res. 2014, 104, 494–503. [Google Scholar] [CrossRef] [Green Version]
- Tellinghausen, J. Least squares with non-normal data: Estimating experimental variance functions. R. Soc. Chem. 2008, 133, 161–166. [Google Scholar] [CrossRef]
- Meltofte, H.; Huntington, H.P.; Barry, T. Introduction. In Arctic Biodiversity Assessment. Status and trends in Arctic biodiversity: Synthesis; Conservation of Arctic Flora and Fauna (CAFF); Meltofte, H., Ed.; Arctic Council: Akureyri, Island, 2013; pp. 9–17. [Google Scholar]
- Convey, P.; Abbandonato, H.D.A.; Bergan, F.; Beumer, L.T.; Biersma, E.M.; Bråthen, V.S.; D’Imperio, L.; Jensen, C.K.; Nilsen, S.; Paquin, K.; et al. Survival of rapidly fluctuating natural low winter temperatures by Arctic soil invertebrates. J. Thermal Biol. 2014, 54, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Cooper, E.J. Warmer shorter winters disrupt Arctic terrestrial ecosystems. Annu. Rev. Ecol. Syst. 2015, 45, 71–95. [Google Scholar] [CrossRef]
- Marshall, W.A. Aerial dispersal of lichen soredia in the maritime Antarctic. New Phytol. 1996, 134, 523–530. [Google Scholar] [CrossRef]
- Bartlett, J.C.; Convey, P.; Pertierra, L.R.; Hayward, S.A.L. An insect invasion of Antarctica: The past, present and future distribution of Eretmoptera murphyi (Diptera, Chironomidae) on Signy Island. Insect Conserv. Div. 2019. [Google Scholar] [CrossRef] [Green Version]
- Cranston, P.S. Eretmoptera murphyi Schaeffer (Diptera: Chironomidae), an apparently parthenogenetic Antarctic midge. Br. Antarct. Surv. Bull. 1985, 66, 35–45. [Google Scholar]
- McDermott, E.G.; Mayo, C.E.; Mullens, B.A. Low temperature tolerance of Culicoides sonorensis (Diptera: Ceratopogonidae) eggs, larvae, and pupae from temperate and subtropical climates. J. Med. Entomol. 2017, 54, 264–274. [Google Scholar]
- Uelmen, J.A.; Duman, J.G.; Lindroth, R.L.; Schwartzberg, E.G.; Raff, K.F. Supercooling points of diapausing forest tent caterpillar (Lepidoptera: Lasiocampidae) eggs. Can. Entomol. 2016, 148, 512–519. [Google Scholar] [CrossRef]
- Tenow, O.; Nilssen, A. Egg cold hardiness and topoclimatic limitations to the outbreaks of Epirrita autumnatain northern Fennoscandia. J. Appl. Ecol. 1990, 27, 723–734. [Google Scholar] [CrossRef]
- Bale, J.S.; Harrington, R.; Clough, M.S. Low temperature mortality of the peach potato aphid Myzus persicae. Ecol. Entomol. 1988, 13, 121–129. [Google Scholar] [CrossRef]
- Kreß, A.; Kuch, U.; Oehlmann, J.; Müller, R. Effects of diapause and cold acclimation on egg ultrastructure: New insights into the cold hardiness mechanisms of the Asian tiger mosquito Aedes (Stegomyia) albopictus. J. Vector. Ecol. 2016, 14, 142–150. [Google Scholar] [CrossRef] [Green Version]
- Ditrich, T.; Koštál, V. Comparative analysis of overwintering physiology in nine species of semi-aquatic bugs (Heteroptera: Gerromorpha). Physiol. Entomol. 2011, 36, 261–270. [Google Scholar] [CrossRef]
- Hughes, K.A.; Worland, M.R. Spatial distribution, habitat preference and colonization status of two alien terrestrial invertebrate species in Antarctica. Antarct. Sci. 2010, 22, 221–231. [Google Scholar] [CrossRef] [Green Version]
- Harada, E.; Lee, R.; Denlinger, D.; Goto, S. Life history traits of adults and embryos of the Antarctic midge Belgica antarctica. Polar Biol. 2014, 37, 1213–1217. [Google Scholar] [CrossRef]
- Sugg, P.; Edwards, J.S.; Baust, J. Phenology and life history of Belgica antarctica, an Antarctic midge (Diptera: Chironomidae). Ecol. Entomol. 1983, 8, 105–113. [Google Scholar] [CrossRef]
- Hayward, S.A.L.; Manso, B.; Cossins, A.R. Molecular basis of chill resistance adaptations in poikilothermic animals. J. Exp. Biol. 2014, 217, 6–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, R.E.; Elnitsky, M.A.; Rinehart, J.P.; Hayward, S.A.; Sandro, L.H.; Denlinger, D.L. Rapid cold-hardening increases the freezing tolerance of the Antarctic midge Belgica antarctica. J. Exp. Bio. 2006, 209, 399–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teets, N.; Dalrymple, E.G.; Hillis, M.H.; Gantz, J.D.; Spacht, D.E.; Lee, R.E.; Denlinger, D.L. Changes in energy reserves and gene expression elicited by freezing and supercooling in the Antarctic midge, Belgica Antarctica. Insects 2020, 11, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teets, N.M.; Kawarasaki, Y.; Lee, R.E.; Denlinger, D.L. Survival and energetic costs of repeated cold exposure in the Antarctic midge, Belgica antarctica: A comparison between frozen and supercooled larvae. J. Exp. Biol. 2011, 214, 806–814. [Google Scholar] [CrossRef] [Green Version]
- Renault, D. Long-term after effects of cold-exposure in adult Alphitobius diaperinus (Tenebrionidae): The need to link survival ability with subsequent reproductive success. Ecol. Entomol. 2011, 36, 36–42. [Google Scholar] [CrossRef]
- Hayward, S.A.L.; Bale, J.S.; Worland, M.R.; Convey, P. Influence of temperature on the hygropreference of the collembolan, Cryptopygus antarcticus, and the mite, Alaskozetes antarcticus from the maritime Antarctic. J. Insect Physiol. 2011, 47, 11–18. [Google Scholar] [CrossRef]
- Elnitsky, M.A.; Benoit, J.B.; Lopez-Martinez, G.; Denlinger, D.L.; Lee, R.E. Osmoregulation and salinity tolerance in the Antarctic midge, Belgica antarctica: Seawater exposure confers enhanced tolerance to freezing and dehydration. J. Exp. Biol. 2009, 212, 2864–2871. [Google Scholar] [CrossRef] [Green Version]
- Matthews, D.H.; Maling, D.H. The geology of the South Orkney Islands: Signy Island. FID Surv. Sci. Rep. 1967, 25, 1–32. [Google Scholar]
- Royles, J.; Griffiths, H. Climate change impacts in polar-regions: Lessons from Antarctic moss bank archives. Global Change Biol. 2014, 21, 1041–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usher, M.B.; Edwards, M. A dipteran from south of the Antarctic Circle: Belgica antarctica (Chironomidae) with a description of its larva. Biol. J. Linn. Soc. 1984, 23, 19–31. [Google Scholar] [CrossRef]






| Tukey’s Multiple Comparisons Test (°C) | Overall Life-Stage Influence p-Value | F Value (1, 1490) | Significant Life-Stage Interactions |
|---|---|---|---|
| 5 vs. −5 | < 0.0001 | 82.31 | Eggs vs. all larvae p < 0.0001 |
| 5 vs. −10 | < 0.0001 | 43.13 | Eggs vs. L2, 3 and 4 p < 0.001 L1 vs. L2 p = 0.02 |
| 5 vs. −15 | < 0.001 | 52.29 | Eggs vs. all larvae p < 0.01 |
| 5 vs. −20 | < 0.001 | 4.28 | Eggs vs. all larvae (eggs only survivors) p < 0.001 |
| Exposure (days) | Dunn’s Multiple Comparisons Test | Mean Rank Diff. | Adjusted p-Value |
|---|---|---|---|
| 28 | C2 vs. C1 | 9.143 | 0.109 |
| C2 vs. NA | 0.8571 | >0.999 | |
| C2 vs. A | 1.714 | >0.999 | |
| 42 | C2 vs. C1 | 14.5 | 0.002 |
| C2 vs. NA | 1.643 | >0.999 | |
| C2 vs. A | 4.143 | >0.999 | |
| 63 | C2 vs. C1 | 18.21 | <0.0001 |
| C2 vs. NA | 11.43 | 0.026 | |
| C2 vs. A | 12.36 | 0.013 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartlett, J.C.; Convey, P.; Hayward, S.A.L. Surviving the Antarctic Winter—Life Stage Cold Tolerance and Ice Entrapment Survival in The Invasive Chironomid Midge Eretmoptera murphyi. Insects 2020, 11, 147. https://doi.org/10.3390/insects11030147
Bartlett JC, Convey P, Hayward SAL. Surviving the Antarctic Winter—Life Stage Cold Tolerance and Ice Entrapment Survival in The Invasive Chironomid Midge Eretmoptera murphyi. Insects. 2020; 11(3):147. https://doi.org/10.3390/insects11030147
Chicago/Turabian StyleBartlett, Jesamine C., Peter Convey, and Scott A. L. Hayward. 2020. "Surviving the Antarctic Winter—Life Stage Cold Tolerance and Ice Entrapment Survival in The Invasive Chironomid Midge Eretmoptera murphyi" Insects 11, no. 3: 147. https://doi.org/10.3390/insects11030147
APA StyleBartlett, J. C., Convey, P., & Hayward, S. A. L. (2020). Surviving the Antarctic Winter—Life Stage Cold Tolerance and Ice Entrapment Survival in The Invasive Chironomid Midge Eretmoptera murphyi. Insects, 11(3), 147. https://doi.org/10.3390/insects11030147