Development, Reproduction, and Life Table Parameters of the Foxglove Aphid, Aulacorthum solani Kaltenbach (Hemiptera: Aphididae), on Soybean at Constant Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insect Colony
2.2. Investigation of Nymph Development and Adult Reproduction at Constant Temperatures
2.3. Models and Estimation of Parameters
2.4. Life Table Construction
3. Results
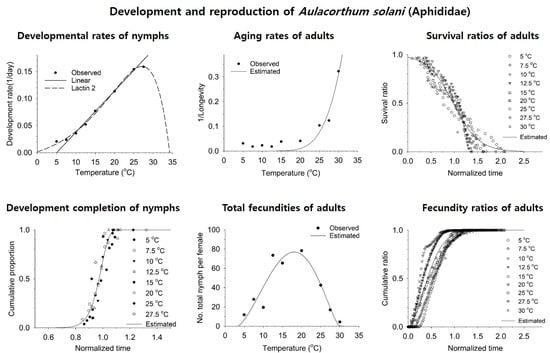
3.1. Nymphal and Adult Development and Fecundity
3.2. Estimation of Developmental and Reproductive Patterns Based on Models
3.3. Life Table
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holman, J. Host Plant Catalog of Aphids: Palaearctic Region; Springer: New York, NY, USA, 2009; pp. 175–185. [Google Scholar]
- Kim, D.-H.; Lee, G.-H.; Park, J.-W.; Hwang, C.-Y. Occurrence aspects and ecological characteristics of foxglove aphid, Aulacorthum solani, Kaltenbach (Homoptera: Aphididae) in soybean. Res. Rept. RDA 1991, 33, 28–32. [Google Scholar]
- Lee, J.S.; Yoo, M.; Jung, J.K.; Bilyeu, K.D.; Lee, J.-D. Detection of novel QTLs for foxglove aphid resistance in soybean. Theor. Appl. Genet. 2015, 128, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Takada, H.; Ono, T.; Torikura, H.; Enokiya, T. Geographic variation in esterase allozymes of Aulacorthum solani (Homoptera: Aphididae) in Japan, in relation to its outbreaks on soybean. Appl. Entomol. Zool. 2006, 41, 595–605. [Google Scholar] [CrossRef] [Green Version]
- Hwang, C.Y.; Uhm, K.B.; Choi, K.M. Seasonal occurrence of aphids (Aulacorthum solani K., Aphis glycines M.) and effects of some insecticides on aphids with infurrow treatment in soybean. Korean J. Plant Prot. 1981, 20, 112–116. [Google Scholar]
- Leather, S.R. Aspects of aphid overwintering (Homoptera: Aphidinea: Aphididae). Entomol. Gener. 1992, 17, 101–113. [Google Scholar] [CrossRef]
- Lee, S.; Holman, J.; Havelka, J. Illustrated Catalogue of Aphididae in the Korean Peninsula. Part I. Subfamily Aphidinae (Hemiptera: Sternorrhyncha); KRIBB & CIS: Chuncheon, Korea, 2002; pp. 106–108. [Google Scholar]
- Miyazaki, M. Forms and morphs of aphids. In Aphids Their Biology, Natural Enemies and Control; Minks, A.K., Harrewijn, P., Eds.; Elsevier: New York, NY, USA, 1987; Volume A, pp. 27–50. [Google Scholar]
- Turl, L.A.D. The effect of winter weather on the survival of aphid populations on weeds in Scotland. EPPO Bull. 1983, 13, 139–143. [Google Scholar] [CrossRef]
- Honěk, A.; Kocourek, F. Temperature and development time in insects: A general relationship between thermal constants. Zool. Jahrbücher Syst. 1990, 117, 401–439. [Google Scholar]
- Chown, S.L.; Nicolson, S.W. Insect Physiological Ecology: Mechanisms and Patterns; Oxford University Press: Oxford, UK, 2004; pp. 115–176. [Google Scholar]
- Damos, P.; Savopoulou-Soultani, M. Temperature-driven models for insect development and vital thermal requirements. Psyche 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Dixon, A.F.G.; Honěk, A.; Keil, P.; Kotela, M.A.A.; Sizling, A.L.; Jarosik, V. Relationship between the minimum and maximum temperature thresholds for development in insects. Funct. Ecol. 2009, 23, 257–264. [Google Scholar] [CrossRef]
- Wagner, T.L.; Wu, H.; Sharpe, P.J.H.; Coulson, R.N. Modeling distribution of insect development time: A literature review and application of Weibull function. Ann. Entomol. Soc. Am. 1984, 77, 475–483. [Google Scholar] [CrossRef]
- De Conti, B.F.; Bueno, V.H.P.; Sampaio, M.V.; Sidney, L.A. Reproduction and fertility life table of three aphid species (Macrosiphini) at different temperatures. Rev. Bras. Entomol. 2010, 54, 654–660. [Google Scholar] [CrossRef] [Green Version]
- De Conti, B.F.; Bueno, V.H.P.; Sampaio, M.V.; Van Lenteren, J.C. Development and survival of Aulacorthum solani, Macrosiphum euphorbiae and Uroleucon ambrosiae at six temperatures. Bull. Insectol. 2011, 64, 63–68. [Google Scholar]
- Lee, S.G.; Kim, H.H.; Kim, T.H.; Park, G.-J.; Kim, K.H.; Kim, J.S. Development model of the foxglove aphid, Aulacorthum solani (Kaltenbach) on lettuce. Korean J. Appl. Entomol. 2008, 47, 359–364. [Google Scholar] [CrossRef]
- Lee, S.G.; Kim, H.H.; Kim, T.H.; Park, G.-J.; Kim, K.H.; Kim, J.S. Longevity and life table of the foxglove aphid (Aulacorthum solani K.) adults on lettuce (Lactuca sativa L.). Korean J. Appl. Entomol. 2008, 47, 365–368. [Google Scholar] [CrossRef]
- Park, G.-J. Temperature-dependent development and its model of the foxglove aphid, Aulacorthum solani (Kaltenbach) (Homoptera: Aphididae). Master’s Thesis, JeonBuk National University, Jeonju, Korea, 22 August 2008. [Google Scholar]
- Jandricic, S.E.; Wraight, S.P.; Bennett, K.C.; Sanderson, J.P. Developmental times and life table statistics of Aulacorthum solani (Hemiptera: Aphididae) at six constant temperatures, with recommendations on the application of temperature-dependent development models. Environ. Entomol. 2010, 39, 1631–1642. [Google Scholar] [CrossRef] [PubMed]
- Kingsolver, J.G.; Higgins, J.K.; Augustine, K.E. Fluctuating temperatures and ectotherm growth: Distinguishing non-linear and time-dependent effects. J. Exp. Biol. 2015, 218, 2218–2225. [Google Scholar] [CrossRef] [Green Version]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variables development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/STAT 9.1 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2004; pp. 1731–1906. [Google Scholar]
- Campbell, A.; Frazer, B.D.; Gilbert, N.; Gutierrrez, A.P.; Markauer, M. Temperature requirements of some aphids and their parasites. J. Appl. Ecol. 1974, 11, 431–438. [Google Scholar] [CrossRef]
- Lactin, D.J.; Holliday, N.J.; Johnson, D.I.; Craigen, R. Improved rate model of temperature-dependent development by arthropods. Environ. Entomol. 1995, 24, 68–75. [Google Scholar] [CrossRef]
- Weibull, W.A. Statistical distribution function of wide applicability. J. Appl. Mech. 1951, 18, 290–293. [Google Scholar]
- Eyring, H. The activated complex in chemical reactions. J. Chem. Phys. 1935, 3, 107–115. [Google Scholar] [CrossRef]
- Curry, G.L.; Feldman, R.M. Mathematical Foundations of Population Dynamics; The Texas A&M University Press: College Station, TX, USA, 1987; pp. 31–76. [Google Scholar]
- Park, J.H.; Kwon, S.H.; Kim, T.O.; Oh, S.O.; Kim, D.-S. Temperature-dependent development and fecundity of Rhopalosiphum padi (L.) (Hemiptera: Aphididae) on corns. Korean J. Appl. Entomol. 2016, 55, 149–160. [Google Scholar] [CrossRef]
- Briere, J.F.; Pracros, P.; LeRoux, A.Y.; Pierre, J.S. A novel rate model of temperature-dependent development for arthropods. Environ. Entomol. 1999, 28, 22–29. [Google Scholar] [CrossRef]
- Kim, D.-S.; Lee, J.-H. Oviposition model of Carposina sasakii (Lepidoptera: Carposinidae). Ecol. Model. 2003, 162, 145–153. [Google Scholar] [CrossRef]
- Sauvion, N.; Rahbé, Y.; Peumans, W.J.; Van Damme, E.J.M.; Gatehouse, J.A.; Gatehouse, A.M.R. Effects of GNA and other mannose binding lectins on development and fecundity of the peach-potato aphid Myzus persicae. Entomol. Exp. Appl. 1996, 79, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Ydergaard, S.; Enkegaard, A.; Brødsgaard, H.F. The predatory mite Hypoaspis miles: Temperature dependent life table characteristics on a diet of sciarid larvae, Bradysia paupera and B. tritici. Entomol. Exp. Appl. 1997, 85, 177–187. [Google Scholar] [CrossRef]
- Birch, L.C. The intrinsic rate of natural increase of an insect population. J. Anim. Ecol. 1948, 17, 15–26. [Google Scholar] [CrossRef]
- Southwood, T.R.E.; Henderson, P.A. Ecological Methods, 3rd ed.; Blackwell Science Ltd.: Oxford, UK, 2000; pp. 404–436. [Google Scholar]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis. 2020. Available online: http://140.120.197.173/Ecology/Download/Twosex-MSChart.exe-B100000.rar (accessed on 19 April 2020).
- Goodman, D. Optimal life histories, optimal notation, and the value of reproductive value. Am. Nat. 1982, 119, 803–823. [Google Scholar] [CrossRef]
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Chapman & Hall: New York, NY, USA, 1993; pp. 39–85. [Google Scholar]
- Chi, H. TIMING-MSChart: A Computer Program for the Population Projection Based on Age-Stage, Two-Sex Life Table. 2020. Available online: http://140.120.197.173/Ecology/Download/Timing-MSChart.rar (accessed on 19 April 2020).
- Pozarowska, B.J. Studies on low temperature survival, reproduction and development in scottish clones of Myzus persicae (Sulzer) and Aulacorthum solani (Kaltenbach) (Hemiptera: Aphididae) susceptible and resistant to organophosphates. Bull. Entomol. Res. 1987, 77, 123–134. [Google Scholar] [CrossRef]
- Kikuchi, T.; Katoh, H.; Kagawa, K.; Sonoda, S.; Murai, T. Effects of high temperatures on development and fecundity of Aulacorthum solani (Homoptera: Aphididae) and Aphis glycines (Homoptera: Aphididae). Jpn. J. Appl. Entomol. Zool. 2018, 62, 41–46. [Google Scholar] [CrossRef]
- Montllor, C.B.; Maxmen, A.; Purcell, A.H. Facultative bacterial endosymbionts benefit pea aphids Acyrthosiphon pisum under heat stress. Ecol. Entomol. 2002, 27, 189–195. [Google Scholar] [CrossRef]
- Awmack, C.S.; Leather, S.R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef] [PubMed]
- Atlihan, R.; Polat-Akkopru, E.; Ozgokce, M.S.; Kasap, I.; Chi, H. Population growth of Dysaphis pyri (Hemiptera: Aphididae) on different pear cultivars with discussion on curve fitting in life table studies. J. Econ. Entomol. 2017, 110, 1890–1898. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-M.; Chi, H.; Wang, R.-C.; Wang, Y.-P.; Xu, Y.-Y.; Li, X.-D.; Yin, P.; Zheng, F.-Q. Demography and uncertainty of population growth of Conogethes punctiferalis (Lepidoptera: Crambidae) reared on five host plants with discussion on some life history statistics. J. Econ. Entomol. 2018, 111, 2143–2152. [Google Scholar] [CrossRef] [PubMed]




| Temp (°C) | First Instar | Second Instar | Third Instar | Fourth Instar | Total Nymph | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| This Study | This Study | This Study | This Study | This Study | Kim et al. [2] 2 | |||||||
| D | M | D | M | D | M | D | M | D | M | D2 | M2 | |
| 2.5 | 16.9 ± 6.2 a 1 | 73.3 | 25.0 ± 16.5 a 1 | 62.5 | - | 100.0 | - | - | - | 100.0 | ||
| 5.0 | 9.3 ± 2.3 b | 3.3 | 12.0 ± 1.3 b | 3.4 | 13.4 ± 1.7 a 1 | 0.0 | 14.3 ± 2.0 a 1 | 3.6 | 48.7 ± 3.4 a 1 | 10.0 | ||
| 7.5 | 8.9 ± 2.2 b | 3.3 | 10.7 ± 1.8 b | 10.3 | 12.0 ± 5.5 a | 3.8 | 12.3 ± 1.6 b | 8.0 | 42.4 ± 5.0 b | 23.3 | ||
| 10.0 | 7.2 ± 1.1 c | 0.0 | 6.7 ± 0.6 c | 3.3 | 6.2 ± 0.4 b | 0.0 | 7.9 ± 0.5 c | 0.0 | 28.0 ± 1.1 c | 3.3 | 20.2 ± 4.5 | 3.0 |
| 12.5 | 4.1 ± 0.5 d | 0.0 | 4.6 ± 0.5 cd | 0.0 | 4.6 ± 0.6 bc | 0.0 | 5.8 ± 0.6 d | 0.0 | 19.2 ± 0.7 d | 0.0 | ||
| 15.0 | 3.2 ± 0.6 de | 0.0 | 3.0 ± 0.3 de | 0.0 | 3.1 ± 0.4 bcd | 0.0 | 3.7 ± 0.5 e | 0.0 | 13.0 ± 0.4 e | 0.0 | 13.4 ± 2.6 | 3.0 |
| 20.0 | 2.2 ± 0.5 ef | 0.0 | 2.1 ± 0.3 e | 0.0 | 2.0 ± 0.3 cd | 0.0 | 2.6 ± 0.5 f | 0.0 | 8.9 ± 0.6 f | 0.0 | 7.8 ± 1.2 | 0.0 |
| 25.0 | 1.7 ± 0.5 f | 0.0 | 1.7 ± 0.5 e | 0.0 | 1.4 ± 0.5 d | 0.0 | 1.8 ± 0.4 g | 0.0 | 6.5 ± 0.6 g | 0.0 | 7.0 ± 1.0 | 0.0 |
| 27.5 | 1.6 ± 0.5 f | 0.0 | 1.4 ± 0.5 e | 0.0 | 1.3 ± 0.5 d | 0.0 | 2.0 ± 0.3 fg | 0.0 | 6.3 ± 0.5 g | 0.0 | ||
| 30.0 | 2.0 ± 0.9 ef | 0.0 | 2.1 ± 0.7 e | 63.3 | 5.0 bc | 90.9 | - | 100.0 | - | 100.0 | - | 100 |
| Temp (°C) | Init. No. 1 | Longevity (Day) | Rep. Rate (%) 3 | R. No. | Pre-Reproductive Period (Day) | Reproductive Period (Day) | Total Fecundity (No.) | Daily Fecundity | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| This Study | Kim et al. [2] 4 | This Study | This Study | This Study | Kim et al. [2] 4 | This Study | Kim et al. [2] 4 | This Study | |||
| 5.0 | 27 | 52.9 ± 30.1 b5 | 92.6 | 25 | 17.4 ± 2.7 a | 22.4 ± 12.1 b | 11.8 ± 5.8 ef | 0.6 ± 0.2 d | |||
| 7.5 | 23 | 67.0 ± 25.3 a | 82.6 | 19 | 12.8 ± 1.4 b | 45.9 ± 19.4 a | 27.8 ± 12.4 d | 0.6 ± 0.2 d | |||
| 10.0 | 29 | 53.2 ± 17.8 b | 52.6 ± 26.7 | 100.0 | 29 | 7.1 ± 0.8 c | 24.5 ± 9.6 b | 34.8 ± 10.4 | 19.4 ± 5.2 de | 41.8 ± 23.6 | 0.9 ± 0.3 d |
| 12.5 | 30 | 65.1 ± 20.4 ab | 100.0 | 30 | 4.6 ± 0.6 d | 46.0 ± 12.6 a | 73.5 ± 12.9 ab | 1.7 ± 0.3 c | |||
| 15.0 | 30 | 33.3 ± 14.3 c | 42.4 ± 21.4 | 100.0 | 30 | 2.6 ± 0.5 e | 28.4 ± 11.9 b | 28.7 ± 8.3 | 65.4 ± 20.9 b | 58.9 ± 25.7 | 2.4 ± 0.4 b |
| 20.0 | 30 | 28.9 ± 9.0 c | 31.4 ± 12.5 | 100.0 | 30 | 1.8 ± 0.4 ef | 21.7 ± 5.9 b | 26.4 ± 5.2 | 78.3 ± 14.6 a | 78.9 ± 19.4 | 3.8 ± 0.6 a |
| 25.0 | 30 | 14.2 ± 7.1 d | 28.2 ± 4.3 | 100.0 | 30 | 1.3 ± 0.5 f | 12.4 ± 6.3 c | 23.8 ± 3.6 | 42.5 ± 18.8 c | 83.0 ± 21.5 | 3.6 ± 0.7 a |
| 27.5 | 30 | 11.5 ± 6.0 d | 93.3 | 28 | 2.5 ± 0.6 e | 7.3 ± 3.6 cd | 16.9 ± 9.3 de | 2.3 ± 0.8 b | |||
| 30.0 2 | 30 | 3.7 ± 1.3 d | 13.3 ± 5.7 | 80.0 | 24 | 1.5 ± 0.6 f | 2.6 ± 1.2 d | 11.2 ± 6.1 | 4.4 ± 2.3 f | 25.4 ± 14.8 | 1.7 ± 0.5 c |
| 35.0 | 4.2 ± 2.1 | 2.3 ± 1.8 | 2.5 ± 1.7 | ||||||||
| Model | Parameters and Derived Estimates | radj2 |
|---|---|---|
| Developmental rate Linear (Equation (1)) | a = 0.00762 ± 0.00026, b = -0.03824 ± 0.00420 (Temperature range 7.5~25 °C), LDT = 5.02, DD = 131.19 | 0.99 |
| Lactin-2 (Equation (2)) | Ρ = 0.14202 ± 0.01970, TL (UDT) = 33.88565 ± 1.61807, ΔT = 7.01425 ± 0.95905, λ = −0.01491 ± 0.01322, LDT = −1.85, Topt = 26.87 | 0.99 |
| Weibull distribution (Equation (3)) | η = 0.99247 ± 0.00444, β = 17.94321 ± 1.87829 | 0.90 |
| Model | Parameters and Derived Values | First Instar | Second Instar | Third Instar | Fourth Instar |
|---|---|---|---|---|---|
| Linear | a (slope) | 0.03351 ± 0.00093 | 0.03579 ± 0.00151 | 0.04072 ± 0.00336 | 0.0298 ± 0.00178 |
| b (intercept) | −0.18052 ± 0.01608 | −0.20268 ± 0.02740 | −0.25756 ± 0.05424 | −0.1702 ± 0.02871 | |
| Temp. range(°C) | 10.0–25.0 | 7.5–27.5 | 7.5–25.0 | 7.5–25.0 | |
| LDT and DD | 5.39, 29.8 | 5.66, 27.9 | 6.32, 24.6 | 5.70, 33.5 | |
| radj.2 | 1.00 | 0.99 | 0.96 | 0.98 | |
| ANOVA | F1,3 = 1308.8 (p < 0.0001) | F1,5 = 565.0 (p < 0.0001) | F1,4 = 146.8 (p = 0.0003) | F1,4 = 281.1 (p < 0.0001) | |
| Lactin-2 | Ρ | 0.14597 ± 0.01197 | 0.18764 ± 0.02063 | 0.23443 ± 0.01455 | 0.19659 ± 0.02063 |
| TL (UDT) | 33.78729 ± 0.60461 | 32.08891 ± 0.51063 | 30.30548 ± 0.11484 | 30.34261 ± 0.60381 | |
| ΔT | 6.75625 ± 0.52066 | 5.30357 ± 0.56751 | 4.25799 ± 0.26086 | 5.06637 ± 0.52048 | |
| λ | −0.024430 ± 0.03485 | 0.02808 ± 0.04283 | 0.06220 ± 0.03000 | 0.01504 ± 0.03185 | |
| LDT | −8.36 | - 1 | - 1 | - 1 | |
| Topt | 27.03 | 26.79 | 26.05 | 25.28 | |
| radj.2 | 0.99 | 0.97 | 0.98 | 0.99 | |
| ANOVA | F3,6 = 409.1 (p < 0.0001) | F3,6 = 121.3 (p < 0.0001) | F3,5 = 188.2 (p < 0.0001) | F3,4 = 214.2 (p < 0.0001) | |
| Weibull | η | 0.96899 ± 0.01653 | 0.94956 ± 0.01867 | 0.91102 ± 0.02093 | 0.96280 ± 0.00995 |
| distribution | β | 3.91272 ± 0.38530 | 7.50896 ± 1.75786 | 5.59675 ± 1.07504 | 10.54906 ± 1.46181 |
| radj.2 | 0.89 | 0.73 | 0.78 | 0.90 | |
| ANOVA | F1,43 = 363.5 (p < 0.0001) | F1,30 = 90.3 (p < 0.0001) | F1,28 = 111.4 (p < 0.0001) | F1,27 = 273.5 (p < 0.0001) |
| Model | Parameters | radj2 |
|---|---|---|
| Aging rate (Equation (4)) | a = 4.70782 ± 7.95564, b = 183.66418 ± 49.24300 | 0.88 |
| Total fecundity (Equation (5)) | a = 0.0074 ± 0.0182, TL = 3.4950 ± 3.0050, TM = 30.0000 ± 3.7360, m = 0.6756 ± 0.3867 | 0.74 |
| Survival ratio (Equation (6)) | γ = 0.97594 ± 0.00988, δ = −0.27363 ± 0.01034 | 0.92 |
| Cumulative fecundity ratio (Equation (3)) | η = 0.51445 ± 0.00655, β = 1.73794 ± 0.05582 | 0.91 |
| Temp (°C) | p1 | p2 | p3 | radj.2 |
|---|---|---|---|---|
| 5.0 | 18.12654 ± 1.31138 | 3.98639 ± 0.68477 | 0.05573 ± 0.00382 | 0.58 |
| 7.5 | 31.87245 ± 1.52136 | 3.74750 ± 0.60615 | 0.04654 ± 0.00221 | 0.70 |
| 10.0 | 22.88171 ± 1.72476 | 1.52812 ± 0.33140 | 0.12175 ± 0.00871 | 0.66 |
| 12.5 | 83.24260 ± 1.99011 | 1.21637 ± 0.20426 | 0.08076 ± 0.00201 | 0.93 |
| 15.0 | 83.47867 ± 2.64907 | 0.22761 ± 0.24133 | 0.10680 ± 0.00374 | 0.91 |
| 20.0 | 88.53896 ± 3.64552 | 0.18145 ± 0.17975 | 0.17008 ± 0.00750 | 0.91 |
| 25.0 | 57.06038 ± 2.09382 | −0.04473 ± 0.12002 | 0.23537 ± 0.00946 | 0.94 |
| 27.5 | 22.04102 ± 1.87325 | 0.30660 ± 0.15650 | 0.30116 ± 0.02514 | 0.81 |
| 30.0 | 4.90767 ± 0.85356 | 0.00785 ± 0.13275 | 0.64841 ± 0.12095 | 0.61 |
| Parameter Estimates | radj.2 |
|---|---|
| ε = −3.42273 ± 0.11850, θ = 0.02574 ± 0.00096, κ = −0.00074 ± 0.00003, ρ = −3.15503 ± 0.06940, ν = 0.00574 ± 0.00057, ψ = −0.00012 ± 0.00002 | 0.90 |
| Temp. (°C) | rm | R0 (No.) | G (Day) | λ | DT (Day) |
|---|---|---|---|---|---|
| 5.0 | 0.0297 | 9.61 | 76.29 | 1.03 | 23.37 |
| 7.5 | 0.0405 | 18.51 | 72.08 | 1.04 | 17.12 |
| 10.0 | 0.0699 | 18.71 | 41.91 | 1.07 | 9.92 |
| 12.5 | 0.1226 | 73.19 | 35.02 | 1.13 | 5.65 |
| 15.0 | 0.1813 | 64.21 | 22.95 | 1.20 | 3.82 |
| 20.0 | 0.2774 | 77.49 | 15.68 | 1.32 | 2.50 |
| 25.0 | 0.3349 | 40.67 | 11.06 | 1.40 | 2.07 |
| 27.5 | 0.2489 | 14.79 | 10.82 | 1.28 | 2.79 |
| Temp. (°C) | rm | R0 (No.) | G (Day) | λ | DT (Day) |
|---|---|---|---|---|---|
| 5.0 | 0.0307 h | 10.51 e | 76.36 a | 1.03 h | 22.61 a |
| 7.5 | 0.0402 g | 18.17 d | 71.85 b | 1.04 g | 17.32 b |
| 10.0 | 0.0689 f | 17.68 d | 41.66 c | 1.07 f | 10.06 c |
| 12.5 | 0.1226 e | 73.56 ab | 35.05 d | 1.13 e | 5.65 d |
| 15.0 | 0.1827 d | 65.29 b | 22.86 e | 1.20 d | 3.79 e |
| 20.0 | 0.2774 b | 78.34 a | 15.72 f | 1.32 b | 2.50 g |
| 25.0 | 0.3377 a | 42.52 c | 11.09 g | 1.40 a | 2.05 h |
| 27.5 | 0.2534 c | 15.79 d | 10.87 g | 1.28 c | 2.74 f |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, B.Y.; Kim, E.Y.; Ahn, J.J.; Kim, Y.; Kang, S.; Jung, J.K. Development, Reproduction, and Life Table Parameters of the Foxglove Aphid, Aulacorthum solani Kaltenbach (Hemiptera: Aphididae), on Soybean at Constant Temperatures. Insects 2020, 11, 296. https://doi.org/10.3390/insects11050296
Seo BY, Kim EY, Ahn JJ, Kim Y, Kang S, Jung JK. Development, Reproduction, and Life Table Parameters of the Foxglove Aphid, Aulacorthum solani Kaltenbach (Hemiptera: Aphididae), on Soybean at Constant Temperatures. Insects. 2020; 11(5):296. https://doi.org/10.3390/insects11050296
Chicago/Turabian StyleSeo, Bo Yoon, Eun Young Kim, Jeong Joon Ahn, Yonggyun Kim, Sungtaeg Kang, and Jin Kyo Jung. 2020. "Development, Reproduction, and Life Table Parameters of the Foxglove Aphid, Aulacorthum solani Kaltenbach (Hemiptera: Aphididae), on Soybean at Constant Temperatures" Insects 11, no. 5: 296. https://doi.org/10.3390/insects11050296
APA StyleSeo, B. Y., Kim, E. Y., Ahn, J. J., Kim, Y., Kang, S., & Jung, J. K. (2020). Development, Reproduction, and Life Table Parameters of the Foxglove Aphid, Aulacorthum solani Kaltenbach (Hemiptera: Aphididae), on Soybean at Constant Temperatures. Insects, 11(5), 296. https://doi.org/10.3390/insects11050296