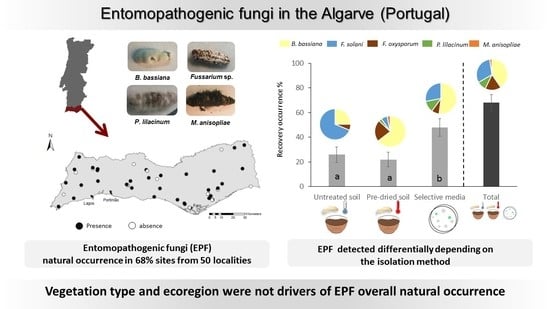
Patterns of Occurrence and Activity of Entomopathogenic Fungi in the Algarve (Portugal) Using Different Isolation Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area, Sampling Method and Soil Parameters
2.2. Entomopathogenic Fungi Isolation
2.3. Fungal Identification
2.4. Comparison of the Detection Methods and Identification of Ecological Drivers for the Entomopathogenic Fungi Natural Occurrence
2.5. Relationships of Entomopathogenic Fungi within Abiotic Factors and Multivariate Analysis of the Entomopathogen Community Assemblage
3. Results
3.1. Distribution of Entomopathogenic Fungi across the Algarve Region: Comparison of Three Methods of Isolation
3.2. Impact of Ecological Drivers on Entomopathogenic Fungi Natural Occurrence
3.3. Abiotic Ranges for the Occurrence of Entomopathogenic Fungi and Patterns of Assemblage into Selected Members of the Entomopathogenic Community
4. Discussion
4.1. Distribution of Entomopathogenic Fungi across the Algarve Region and Comparison of Three Isolation Methods
4.2. Entomopathogenic Fungi Species Composition and the Ecological Drivers of Their Natural Occurrence
4.3. Entomopathogenic Fungi Assemblage Patterns
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bidochka, M.J.; Kasperski, J.E.; Wild, G.A.M. Occurrence of the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana in soils from temperate and near-northern habitats. Can. J. Bot. 1998, 76, 1198–1204. [Google Scholar]
- Meyling, N.V.; Eilenberg, J. Occurrence and distribution of soil borne entomopathogenic fungi within a single organic agroecosystem. Agric. Ecosyst. Environ. 2006, 113, 336–341. [Google Scholar] [CrossRef]
- McGuire, A.M.; Northfield, T.D. Tropical Occurrence and Agricultural Importance of Beauveria bassiana and Metarhizium anisopliae. Front. Sustain. Food Syst. 2020, 4. [Google Scholar] [CrossRef] [Green Version]
- Charnley, A.K.; Collins, S.A. Entomopathogenic fungi and their role in pest control. In Environmental and Microbial Relationships, 2nd ed.; Kubicek, C.P., Druzhinina, I.S., Eds.; The Mycota IV; Springer: Berlin/Heidelberg, Germany, 2007; pp. 159–187. [Google Scholar]
- Oreste, M.; Bubici, G.; Poliseno, M.; Triggiani, O.; Tarasco, E. Pathogenicity of Beauveria bassiana (Bals.-Criv.) Vuill. and Metarhizium anisopliae (Metschn.) sorokin against Galleria mellonella L. and Tenebrio molitor L. in laboratory assays. REDIA J. Zool. 2012, 95, 43–48. [Google Scholar]
- Altinok, H.H.; Altinok, M.A.; Koca, A.S. Modes of Action of Entomopathogenic Fungi. Curr. Trends Nat. Sci. 2019, 8, 117–124. [Google Scholar]
- Strasser, H.; Abendstein, D.; Stuppner, H.; Butt, T.M. Monitoring the distribution of secondary metabolites produced by the entomogenous fungus Beauveria brongniartii with particular reference to oosporein. Mycol. Res. 2000, 104, 1227–1233. [Google Scholar] [CrossRef]
- Donatti, A.C.; Furlaneto-Maia, L.; Fungaro, M.H.; Furlaneto, M.C. Production and regulation of cuticle-degrading proteases from Beauveria bassiana in the presence of Rhammatocerus schistocercoides cuticle. Cur. Microbiol. 2008, 56, 256–260. [Google Scholar] [CrossRef]
- Vega, F.E.; Dowd, P.F.; Lacey, L.A.; Pell, J.K.; Jackson, D.M.; Klein, M.G. Dissemination of beneficial microbial agents by insects. In Field Manual of Techniques in Invertebrate Pathology; Lacey, L.A., Kaya, H.K., Eds.; Academic Press: Dordrecht, The Netherland, 2007; pp. 153–177. [Google Scholar]
- Bueno-Pallero, F.A.; Blanco-Pérez, R.; Dionisio, L.; Campos-Herrera, R. Simultaneous exposure of nematophagous fungi, entomopathogenic nematodes and entomopathogenic fungi can modulate belowground insect pest control. J. Invertebr. Pathol. 2018, 154, 85–94. [Google Scholar] [CrossRef]
- Zimmerman, G. The Galleria bait method for detection of entomopathogenic fungi in soil. J. Appl. Entomol. 1986, 102, 213–215. [Google Scholar] [CrossRef]
- Meyling, N.V. Methods for isolation of entomopathogenic fungi from the soil environment. Man. Isol. Soil Borne Entomopathog. Fungi 2007, 1–18. Available online: www.orgprints.org/11200 (accessed on 17 December 2019).
- Uzman, D.; Pliester, J.; Leyer, I.; Entling, M.H.; Reineke, A. Drivers of entomopathogenic fungi presence in organic and conventional vineyard soils. Appl. Soil Ecol. 2018, 133, 89–97. [Google Scholar] [CrossRef]
- Goble, T.A. Investigation of Entomopathogenic Fungi for Control of False Codling Moth, Thaumatotibia leucotrata, Mediterranean Fruit Fly, Ceratitis Capitata and Natal Fruit Fly, C. rosa in South African Citrus. Master’s Thesis, Rhodes University, Grahamstown, South Africa, 2010. Available online: http://hdl.handle.net/10962/d1005409 (accessed on 16 May 2013).
- Sánchez-Peña, S.R.; San-Juan Lara, J.; Medina, R.F. Occurrence of entomopathogenic fungi from agricultural and natural ecosystems in Saltillo, México, and their virulence towards thrips and whiteflies. J. Insect Sci. 2011, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Meyling, N.V.; Schmidt, N.M.; Eilenberg, J. Occurrence and diversity of fungal entomopathogens in soils of low and high Arctic Greenland. Polar Biol. 2012, 35, 1439–1445. [Google Scholar] [CrossRef]
- Steinwender, B.M.; Enkerli, J.; Widmer, F.; Eilenberg, J.; Thorup-Kristensen, K.; Meyling, N.V. Molecular diversity of the entomopathogenic fungal Metarhizium community within an agroecosystem. J. Invertebr. Pathol. 2014, 123, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Oliveira, I.; Torres, L.; Marques, G. Entomopathogenic fungi in Portuguese vineyards soils: Suggesting a ‘Galleria-Tenebrio-bait method’ as bait insects Galleria and Tenebrio significantly underestimate the respective recoveries of Metarhizium (robertsii) and Beauveria (bassiana). MycoKeys 2018, 38, 1–23. [Google Scholar] [CrossRef]
- Meyling, N.V.; Eilenberg, J. Ecology of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in temperate agroecosystems: Potential for conservation biological control. Biol. Control 2007, 43, 145–155. [Google Scholar] [CrossRef]
- Campos-Herrera, R.; Lacey, L. Methods for studying the ecology of invertebrate diseases and pathogen. In Ecology of Invertebrate Diseases; Hajek, A.E., Shapiro-Ilan, D., Eds.; Wiley: Hoboken, NJ, USA, 2018; pp. 19–48. [Google Scholar]
- Fernandes, E.K.K.; Keyser, C.A.; Rangel, D.E.N.; Foster, R.N.; Roberts, D.W. CTC medium: A novel dodine-free selective medium for isolating entomopathogenic fungi, especially Metarhizium acridum, from soil. Biol. Control 2010, 54, 197–205. [Google Scholar] [CrossRef]
- Shin, T.Y.; Choi, J.B.; Bae, S.M.; Koo, H.M.; Woo, S.D. Study on selective media for isolation of entomopathogenic fungi. Int. J. Indust. Entomol. 2010, 20, 7–12. [Google Scholar]
- Inglis, G.D.; Enkerli, J.; Goettel, M.S. Laboratory techniques used for entomopathogenic fungi: Hypocreales. In Manual of Techniques in Invertebrate Pathology, 2nd ed.; Lacey, L.A., Ed.; Academic Press/Elsevier: San Diego, CA, USA, 2012; pp. 253–285. [Google Scholar]
- Shapiro-Ilan, D.I.; Gardner, W.A.; Wells, L.; Wood, B.W. Cumulative impact of a clover cover crop on the persistence and efficacy of Beauveria bassiana in suppressing the pecan weevil (Coleoptera: Curculionidae). Environ. Entomol. 2012, 41, 298–307. [Google Scholar] [CrossRef]
- Korosi, G.A.; Wilson, B.A.L.; Powell, K.S.; Ash, G.J.; Reineke, A.; Savocchia, S. Occurrence and diversity of entomopathogenic fungi (Beauveria spp. and Metarhizium spp.) in Australian vineyard soils. J. Invertebr. Pathol. 2019, 164, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Hibbett, D.S.; Binder, M.; Bishoff, J.F.; Blackwell, M.; Cannon, P.F.; Eriksson, O.E.; Huhndorf, S.; James, T.; Kirk, P.M.; Lucking, R.; et al. A higher-level phylogenetic classification of the Fungi. Mycol. Res. 2007, 111, 509–547. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Rehner, S.A.; Widmer, F.; Enkerli, J. A PCR-based tool for cultivation-independent detection and quantification of Metarhizium clade 1. J. Invertebr. Pathol. 2011, 108, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Inglis, G.D.; Goettel, M.S.; Butt, T.M.; Strasser, H. Use of hyphomycetous fungi for managing insect pests. In Fungi as Biocontrol Agents; Progress, Problems and Potential; Butt, T.M., Jackson, C., Magan, N., Eds.; CABI Publishing: Wallingford, UK, 2001; pp. 23–69. [Google Scholar]
- Hajek, A.E.; St. Leger, R.J. Interactions between fungal pathogens and insect hosts. Ann. Rev. Entomol. 1994, 39, 293–322. [Google Scholar] [CrossRef]
- McLaughlin, D.J.; Hibbett, D.S.; Lutzoni, F.; Spatafora, J.W.; Vilgalys, R. The search for the fungal tree of life. Evol. Microbiol. 2009, 17, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Wall, D. Soil Ecology and Ecosystem Services; Oxford University Press: Oxford, UK, 2012; p. 406. [Google Scholar]
- Jeffs, L.B.; Khachatourians, G.G. Toxic properties of Beauveria pigments on erythrocyte membranes. Toxicon 1997, 35, 1351–1356. [Google Scholar] [CrossRef]
- Jaronski, S.T. Ecological factors in the inundative use of fungal Entomopathogens. BioControl 2010, 55, 159–185. [Google Scholar] [CrossRef]
- Asensio, L.; Carbonell, T.; López-Jiméwnez, J.A.; López-Llorca, L.V. Entomopathogenic fungi in soils from Alicante province. Span. J. Agric. Res. 2003, 3, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Jaronski, S.T. Soil ecology of the entomopathogenic ascomycetes: A critical examination of what we (think) we know. In Use of Entomopathogenic Fungi in Biological Pest Management; Research Signpost: Trivandrum, Kerala, India, 2007; pp. 91–144. [Google Scholar]
- Eilenberg, J.; Hokkanen, H.M.T. An Ecological and Societal Approach to Biological Control, Degeneration of Entomogenous Fungi, Progress in Biological Control; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Thiele-Bruhn, S.; Bloem, J.; de Vries, F.T.; Kalbitz, K.; Wagg, C. Linking soil biodiversity and agricultural soil management. Curr. Opin. Environ. Sustain. 2012, 4, 523–528. [Google Scholar] [CrossRef]
- Shapiro-Ilan, D.A.; Jackson, J.; Reilly, C.C.; Hotchkiss, M.W. Effects of combining an entomopathogenic fungi or bacterium with entomopathogenic nematodes on mortality of Curculio caryae (Coleoptera: Curculionidae). Biol. Control 2004, 30, 119–126. [Google Scholar] [CrossRef]
- Jabbour, R.; Crowder, D.W.; Aultman, E.A.; Snyder, W.E. Entomopathogen biodiversity increases host mortality. Biol. Control 2011, 59, 277–283. [Google Scholar] [CrossRef]
- Hajek, A.E.; Meyling, N.V. Fungi. In Ecology of Invertebrate Diseases; Hajek, A.E., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2018; pp. 327–377. [Google Scholar]
- Shapiro-Ilan, D.; Brown, I. Earthworms as phoretic hosts for Steinernema carpocapsae and Beauveria bassiana: Implications for enhanced biological control. Biol. Control 2013, 66, 41–48. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; Navas-Cortes, J.A.; Maranhao, E.A.; Ortiz-Urquiza, A.; Santiago-Alvarez, C. Factors affecting the occurrence and distribution of entomopathogenic fungi in natural and cultivated soils. Mycol. Res. 2007, 111, 947–966. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.; Pereira, J.A.; Quesada-Moraga, E.; Lino-Neto, T.; Bento, A.; Baptista, P. Effect of soil tillage on natural occurrence of fungal entomopathogens associated to Prays oleae Bern. Sci. Hortic. 2013, 159, 190–196. [Google Scholar] [CrossRef]
- Garrido-Jurado, I.; Fernández-Bravo, M.; Campos, C.; Quesada-Moraga, E. Diversity of entomopathogenic Hypocreales in soil and phylloplanes of five Mediterranean cropping systems. J. Invertebr. Pathol. 2015, 130, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Campos-Herrera, R.; Blanco-Pérez, R.; Bueno-Pallero, F.A.; Duarte, A.; Nolasco, G.; Sommer, R.J.; Rodríguez Martín, J.A. Vegetation drives assemblages of entomopathogenic nematodes and other soil organisms: Evidence from the Algarve, Portugal. Soil Biol. Biochem. 2019, 128, 150–163. [Google Scholar] [CrossRef]
- Foth, H.D. Fundamentals of Soil Science; John Wiley & Sons: London, UK, 1984. [Google Scholar]
- Shapiro-Ilan, D.I.; Gardner, W.A.; Fuxa, J.R.; Wood, B.W.; Nguyen, K.B.; Adams, B.J.; Humber, R.A.; Hall, M.J. Survey of entomopathogenic nematodes and fungi endemic to pecan orchards of the Southeastern United States and their virulence to the Pecan Weevil (Coleoptera: Curculionidae). Environ. Entomol. 2003, 32, 187–195. [Google Scholar] [CrossRef]
- Klingen, I.; Meadow, R.; Aandal, T. Mortality of Delia floralis, Galleria mellonella and Mamestra brassicae treated with insect pathogenic hyphomycetous fungi. J. Appl. Entomol. 2002, 126, 231–237. [Google Scholar] [CrossRef]
- Cabrera-Mora, J.A.; Guzmán-Franco, A.W.; Santillán-Galicia, M.T.; Tamayo-Mejía, F. Niche separation of species of entomopathogenic fungi within the genera Metarhizium and Beauveria in different cropping systems in Mexico. Fungal Ecol. 2019, 39, 349–355. [Google Scholar] [CrossRef]
- Barnett, L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi, 4th ed.; MacMillan Publishing: New York, NY, USA, 1987; p. 218. [Google Scholar]
- Humber, R.A. Fungi: Identification. In Manual of Techniques in Insect Pathology; Lacey, L.A., Ed.; Academic Press: San Diego, CA, USA, 1997; pp. 153–185. [Google Scholar]
- Humber, R.A. Entomopathogenic Fungal Identification; US Plant, Soil & Nutrition Laboratory: New York, NY, USA, 2005.
- White, G.F. A method for obtaining infective nematode larvae from cultures. Science 1927, 66, 302–303. [Google Scholar] [CrossRef]
- Luo, G.; Mitchell, T.G. Rapid identification of pathogenic fungi directly from cultures by using multiplex PCR. J. Clin. Microbiol. 2002, 40, 2860–2865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ter Braak, C.J.F. Biometris e Quantitative Methods in the Life and Earth Sciences; Plant Research International, Wageningen University and Research Centre: Wageningen, The Netherlands, 2009. [Google Scholar]
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using CANOCO, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014; Volume 5, p. 373. [Google Scholar]
- Lepš, J.; Hadincová, V. How reliable are our vegetation analyses? J. Veg. Sci. 1992, 3, 119–124. [Google Scholar] [CrossRef]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003; p. 269. [Google Scholar]
- Bruck, D.J. Natural occurrence of entomopathogens in Pacific Northwest nursery soils and their virulence to the black vine weevil, Otiorhynchus sulcatus (F.) (Coleoptera: Curculionidae). Environ. Entomol. 2004, 33, 1335–1343. [Google Scholar] [CrossRef] [Green Version]
- Rath, A.C.; Koen, T.B.; Yip, H.Y. The influence of abiotic factors on the distribution and abundance of Metarhizium anisopliae in Tasmanian pasture soils. Mycol. Res. 1992, 96, 378–384. [Google Scholar] [CrossRef]
- Chandler, D.; Mietkiewski, R.T.; Davidson, G.; Pell, J.K. Impact of habitat type and pesticide application on the natural occurrence of entomopathogenic fungi in UK soils. OILB WPRS Bull. 1998, 1, 81–84. [Google Scholar]
- Sevim, A.; Demir, I.; Höfte, M.; Humber, R.A.; Demirbag, Z. Isolation and characterization of entomopathogenic fungi from hazelnut-growing region of Turkey. BioControl 2009, 55, 279–297. [Google Scholar] [CrossRef]
- Keller, S.; Kessler, P.; Schweizer, C. Distribution of insect pathogenic soil fungi in Switzerland with special reference to Beauveria brongniartii and Metarhizium anisopliae. BioControl 2003, 48, 307–319. [Google Scholar] [CrossRef]
- Hominick, W.M. Biogeography. In Entomopathogenic Nematology; Gaugler, R., Ed.; CABI Publishing: Wallingford, UK, 2002; pp. 115–143. [Google Scholar]
- Mietkiewski, R.; Tkaczuk, C. The spectrum and frequency of entomopathogenic fungi in litter, forest soil and arable soil. IOBC/WPRS Bull. 1998, 21, 41–44. [Google Scholar]
- Kessler, P.; Matzke, H.; Keller, S. The effect of application time and soil factors on the occurrence of Beauveria brongniartii applied as a biological control agent in soil. J. Invertebr. Pathol. 2003, 84, 15–23. [Google Scholar] [CrossRef]
- El-Ghany, A.T.M.; El-Sheikh, H.H.; El-Rahman, A.G.A.; El-Nasser, A. Biodiversity of entomopathogenic fungi in new cultivated soil with their using to control of Galleria mellonella. Int. J. Cur. Res. Rev. 2012, 4, 17–31. [Google Scholar]
- Wu, S.Y.; El-Borai, F.E.; Graham, J.H.; Duncan, L.W. The saprophytic fungus Fusarium solani increases the insecticidal efficacy of the entomopathogenic nematode Steinernema diaprepesi. J. Invertebr. Pathol. 2018, 159, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Marques, G. Fusarium, an Entomopathogen—A Myth or Reality? Pathogens 2018, 7, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos-Herrera, R.; Rodríguez Martín, J.A.; Escuer, M.; García-González, M.T.; Duncan, L.W.; Gutiérrez, C. Entomopathogenic nematode food webs in an ancient, mining pollution gradient in Spain. Sci. Tot. Environ. 2016, 572, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, R.; Barbercheck, M.E. Soil management effects on entomopathogenic fungi during the transition to organic agriculture in a feed grain rotation. Biol. Control. 2009, 51, 435–443. [Google Scholar] [CrossRef]
- Imperiali, N.; Chiriboga, M.X.; Schlaeppi, K.; Fesselet, M.; Villacrés, D.; Jaffuel, G.; Bender, S.F.; Dennert, F.; Blanco-Pérez, R.; van der Heijden, M.G.A.; et al. Combined field inoculations of Pseudomonas bacteria, arbuscular mycorrhizal fungi and entomopathogenic nematodes and their effects on wheat performance. Front. Plant. Sci. 2017, 8, 1809. [Google Scholar] [CrossRef] [Green Version]
- Jaffuel, G.; Imperiali, N.; Shelby, K.; Campos-Herrera, R.; Geisert, R.; Maurhofer, M.; Loper, J.; Keel, C.; Turlings, T.C.J.; Hibbard, B.E. Protecting maize from rootworm damage with the combined application of arbuscular mycorrhizal fungi, Pseudomonas bacteria and entomopathogenic nematodes. Sci. Rep. 2019, 9, 3127. [Google Scholar] [CrossRef]





| Entomopathogenic Fungi Species | |||||
|---|---|---|---|---|---|
| Variables for Characterization | Beauveria bassiana | Fusarium solani | Fusarium oxysporum | Purpureocillium lilacinum | Metarhizium anisopliae |
| General characteristics | |||||
| Isolation method a | U, D, S | U, D, S | U, D, S | D, S | D |
| No. positive sites | 17 (34%) | 13 (26%) | 7 (14%) | 4 (8%) | 1 (2%) |
| GenBank accession number | MN808334 | MN808329, MN808331 | MN808330, MN808332 | MN808335 | MN808333 |
| General properties | |||||
| Habitat type | oak, pine, palmetto, citrus | oak, pine, palmetto, citrus | oak, pine, citrus | oak, pine | oak |
| Ecoregion | calcareous, non-calcareous | calcareous, non-calcareous | calcareous, non-calcareous | non-calcareous | non-calcareous |
| Altitude (m ASL) | 23–527 | 9–500 | 4–527 | 99–215 | 99 |
| pH | 5.0–8.1 | 4.9–8.1 | 5.1–8.0 | 5.1–6.1 | 6.1 |
| CE (µS/cm) | 65–523 | 82–430 | 65–402 | 66–374 | 374 |
| SOM (%) | 1.6–11.1 | 2.3–17.6 | 1.8–10.8 | 3.8–10.8 | 10.8 |
| Sand (%) | 25–87 | 25–80 | 25–80 | 26–47 | 33 |
| Silt (%) | 10–44 | 7–49 | 15–39 | 31–43 | 39 |
| Clay (%) | 3–48 | 3–37 | 5–39 | 18–35 | 28 |
| Soil fertility | |||||
| P (mg·kg−1) | 0.02–47.5 | 0.17–49.1 | 0.02–25.2 | 0.02–3.9 | 0.01 |
| K (mg·kg−1) | 23–110 | 25–165 | 25–141 | 25–127 | 127 |
| Mg (mg·kg−1) | 49–1160 | 4–1068 | 49–746 | 220–646 | 646 |
| Ca (mg·kg−1) | 529–3867 | 545–4851 | 542–2589 | 584–1510 | 1510 |
| Zn (mg·kg−1) | 0.04–7.22 | 0.03–9.60 | 0.06–4.73 | 0.90–3.10 | 3.11 |
| Mn (mg·kg−1) | 7.7–34.6 | 2.7–35.0 | 11.5–34.6 | 33.5–34.6 | 34.6 |
| Fe (mg·kg−1) | 3.8–76.7 | 0.9–67.9 | 3.3–62.1 | 40.3–73.7 | 40.3 |
| Cu (mg·kg−1) | 0.01–4.97 | 0.02–8.13 | 0.01–2.75 | 0.5–1.6 | 1.40 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bueno-Pallero, F.Á.; Blanco-Pérez, R.; Vicente-Díez, I.; Rodríguez Martín, J.A.; Dionísio, L.; Campos-Herrera, R. Patterns of Occurrence and Activity of Entomopathogenic Fungi in the Algarve (Portugal) Using Different Isolation Methods. Insects 2020, 11, 352. https://doi.org/10.3390/insects11060352
Bueno-Pallero FÁ, Blanco-Pérez R, Vicente-Díez I, Rodríguez Martín JA, Dionísio L, Campos-Herrera R. Patterns of Occurrence and Activity of Entomopathogenic Fungi in the Algarve (Portugal) Using Different Isolation Methods. Insects. 2020; 11(6):352. https://doi.org/10.3390/insects11060352
Chicago/Turabian StyleBueno-Pallero, Francisco Ángel, Rubén Blanco-Pérez, Ignacio Vicente-Díez, José Antonio Rodríguez Martín, Lídia Dionísio, and Raquel Campos-Herrera. 2020. "Patterns of Occurrence and Activity of Entomopathogenic Fungi in the Algarve (Portugal) Using Different Isolation Methods" Insects 11, no. 6: 352. https://doi.org/10.3390/insects11060352
APA StyleBueno-Pallero, F. Á., Blanco-Pérez, R., Vicente-Díez, I., Rodríguez Martín, J. A., Dionísio, L., & Campos-Herrera, R. (2020). Patterns of Occurrence and Activity of Entomopathogenic Fungi in the Algarve (Portugal) Using Different Isolation Methods. Insects, 11(6), 352. https://doi.org/10.3390/insects11060352