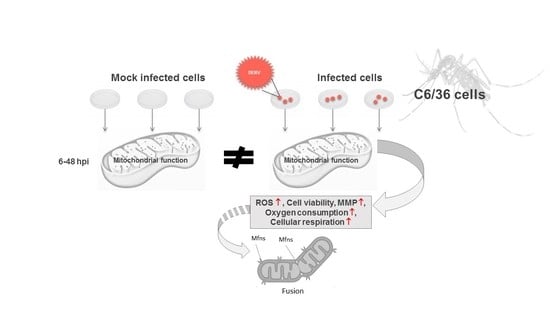
Monitoring Mitochondrial Function in Aedes albopictus C6/36 Cell Line during Dengue Virus Infection
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Virus
2.2. ROS Measurement
2.3. Cell Viability Assay
2.4. ATP Measurement
2.5. Mitochondrial Membrane Potential Assay
2.6. Oxygen Consumption Measurement
2.7. Immunofluorescence
2.8. Statistical Analysis
3. Results
3.1. DENV2 Infection Induces Oxidative Stress in C6/36 Cells
3.2. DENV2 Infection Does Not Alter the General Energetic Status of C6/36 Cells
3.3. Mitochondrial Respiration in C6/36 Cells during DENV2 Infection
3.4. Changes in Mitochondrial Function Are Not Related to Mitochondrial Fission
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bratic, I.; Trifunovic, A. Biochimica et Biophysica Acta Mitochondrial energy metabolism and ageing. Biochim. Biophys. Acta (BBA) Bioenerg. 2010, 1797, 961–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letts, J.; Fiedorczuk, K.; Sazanov, L. The architecture of respiratory supercomplexes. Nature 2016, 537, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Irina, B.; Jankauskas, S.S.; Federation, R. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2008, 417, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [Green Version]
- Youle, R.J.; Van Der Bliek, A.M. Mitochondrial Fission, Fusion, and Stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef] [Green Version]
- Tilokani, L.; Nagashima, S.; Paupe, V.; Prudent, J. Mitochondrial dynamics: Overview of molecular mechanisms. Essays Biochem. 2018, 62, 341–360. [Google Scholar] [CrossRef] [Green Version]
- Kraemer, M.U.G.; Reiner, R.C., Jr.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef]
- Rodenhuis-Zybert, I.; Wilschut, J.; Smit, J.M. Dengue virus life cycle: Viral and host factors modulating infectivity. Cell. Mol. Life Sci. 2010, 67, 2773–2786. [Google Scholar] [CrossRef]
- Welsch, S.; Miller, S.; Brey, I.R.; Merz, A.; Bleck, C.; Walther, P.; Fuller, S.D.; Antony, C.; Krijnse-Locker, J.; Bartenschlager, R. Composition and Three-Dimensional Architecture of the Dengue Virus Replication and Assembly Sites. Cell Host Microbe 2009, 5, 365–375. [Google Scholar] [CrossRef] [Green Version]
- Chatel-Chaix, L.; Bartenschlager, R. Dengue Virus- and Hepatitis C Virus-Induced Replication and Assembly Compartments: The Enemy Inside--Caught in the Web. J. Virol. 2014, 88, 5907–5911. [Google Scholar] [CrossRef] [Green Version]
- Chatel-Chaix, L.; Cortese, M.; Brey, I.R.; Bender, S.; Neufeldt, C.; Fischl, W.; Scaturro, P.; Schieber, N.; Schwab, Y.; Fischer, B.; et al. Dengue Virus Perturbs Mitochondrial Morphodynamics to Dampen Innate Immune Responses. Cell Host Microbe 2016, 20, 342–356. [Google Scholar] [CrossRef] [Green Version]
- Barbier, V.; Lang, D.; Valois, S.; Rothman, A.; Medin, C.L. Dengue virus induces mitochondrial elongation through impairment of Drp1-triggered mitochondrial fission. Virology 2016, 500, 149–160. [Google Scholar] [CrossRef] [PubMed]
- El-Bacha, T.; Midlej, V.D.V.P.; da Silva, A.P.P.; da Costa, L.S.; Benchimol, M.; Galina, A.; Da Poian, A. Mitochondrial and bioenergetic dysfunction in human hepatic cells infected with dengue 2 virus. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2007, 1772, 1158–1166. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.-Y.; Liang, J.-J.; Li, J.-K.; Lee, Y.-L.; Chang, B.-L.; Su, C.-I.; Huang, W.-J.; Lai, M.M.C.; Lin, Y.-L. Dengue Virus Impairs Mitochondrial Fusion by Cleaving Mitofusins. PLoS Pathog. 2015, 11, e1005350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez, R.R.; Ludert, J.E. The Dengue Virus Nonstructural Protein 1 (NS1) Is Secreted from Mosquito Cells in Association with the Intracellular Cholesterol Transporter Chaperone Caveolin Complex. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [Green Version]
- Brand, M.D.; Nicholls, D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziviani, E.; Tao, R.N.; Whitworth, A.J. Drosophila Parkin requires PINK1 for mitochondrial translocation and ubiquitinates Mitofusin. Proc. Natl. Acad. Sci. USA 2010, 107, 5018–5023. [Google Scholar] [CrossRef] [Green Version]
- Poole, A.; Thomas, R.E.; Yu, S.; Vincow, E.S.; Pallanck, L. The Mitochondrial Fusion-Promoting Factor Mitofusin Is a Substrate of the PINK1/Parkin Pathway. PLoS ONE 2010, 5, e10054. [Google Scholar] [CrossRef] [Green Version]
- Roy, J.; Galano, J.; Durand, T.; Le Guennec, J.; Lee, J.C. Physiological role of reactive oxygen species as promoters of natural defenses. FASEB J. 2017, 31, 3729–3745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeJong, R.J.; Miller, L.M.; Molina-Cruz, A.; Gupta, L.; Kumar, S.; Barillas-Mury, C. Reactive oxygen species detoxification by catalase is a major determinant of fecundity in the mosquito Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2007, 104, 2121–2126. [Google Scholar] [CrossRef] [Green Version]
- Ha, E.-M.; Oh, C.-T.; Bae, Y.S.; Lee, W.-J. A Direct Role for Dual Oxidase in Drosophila Gut Immunity. Science 2005, 310, 847–850. [Google Scholar] [CrossRef]
- Kumar, S.; Molina-Cruz, A.; Gupta, L.; Rodrigues, J.; Barillas-Mury, C. A Peroxidase/Dual Oxidase System Modulates Midgut Epithelial Immunity in Anopheles gambiae. Science 2010, 327, 1644–1648. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.-H.; Tang, P.; Yang, C.-F.; Kao, L.-H.; Lo, Y.-P.; Chuang, C.-K.; Shih, Y.-T.; Chen, W.-J. Antioxidant defense is one of the mechanisms by which mosquito cells survive dengue 2 viral infection. Virology 2011, 410, 410–417. [Google Scholar] [CrossRef] [Green Version]
- Heiden, M.G.; Chandel, N.S.; Schumacker, P.T.; Thompson, C.B. Bcl-xL Prevents Cell Death following Growth Factor Withdrawal by Facilitating Mitochondrial ATP/ADP Exchange. Mol. Cell 1999, 3, 159–167. [Google Scholar] [CrossRef]
- Kim, S.-J.; Ahn, D.-G.; Syed, G.H.; Siddiqui, A. The essential role of mitochondrial dynamics in antiviral immunity. Mitochondrion 2017, 41, 21–27. [Google Scholar] [CrossRef]
- Khan, M.; Syed, G.; Kim, S.-J.; Siddiqui, A. Mitochondrial dynamics and viral infections: A close nexus. Biochim. Biophys. Acta (BBA) Bioenerg. 2015, 1853, 2822–2833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karpf, A.; Lenches, E.; Strauss, E.G.; Strauss, J.H.; Brown, D.T. Superinfection exclusion of alphaviruses in three mosquito cell lines persistently infected with Sindbis virus. J. Virol. 1997, 71, 7119–7123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olagnier, D.; Peri, S.; Steel, C.; Van Montfoort, N.; Chiang, C.; Beljanski, V.; Slifker, M.; He, Z.; Nichols, C.N.; Lin, R.; et al. Cellular Oxidative Stress Response Controls the Antiviral and Apoptotic Programs in Dengue Virus-Infected Dendritic Cells. PLoS Pathog. 2014, 10, e1004566. [Google Scholar] [CrossRef] [PubMed]
- Molina-Cruz, A.; DeJong, R.J.; Charles, B.; Gupta, L.; Kumar, S.; Jaramillo-Gutierrez, G.; Barillas-Mury, C. Reactive Oxygen Species Modulate Anopheles gambiae Immunity against Bacteria and Plasmodium. J. Biol. Chem. 2008, 283, 3217–3223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goncalves, R.L.S.; Oliveira, J.H.M.; Oliveira, G.A.; Andersen, J.F.; Oliveira, M.F.; Oliveira, P.L.; Barillas-Mury, C. Mitochondrial Reactive Oxygen Species Modulate Mosquito Susceptibility to Plasmodium Infection. PLoS ONE 2012, 7, e41083. [Google Scholar] [CrossRef] [Green Version]
- Ha, E.-M.; Oh, C.-T.; Ryu, J.-H.; Bae, Y.-S.; Kang, S.-W.; Jang, I.-H.; Brey, P.T.; Lee, W.-J. An Antioxidant System Required for Host Protection against Gut Infection in Drosophila. Dev. Cell 2005, 8, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Wei, G.; Lai, Y.; Wang, G.; Chen, H.; Li, F.; Wang, S. Insect pathogenic fungus interacts with the gut microbiota to accelerate mosquito mortality. Proc. Natl. Acad. Sci. USA 2017, 114, 5994–5999. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Yang, L.; Pang, X.; Zhag, R.; Zhu, Y.; Wang, P.; Gao, G.; Cheng, G. A Mesh–Duox pathway regulates homeostasis in the insect gut. Nat. Microbiol. 2017, 2, 17020. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zhou, G.; Wu, J.; Bian, G.; Lu, P.; Raikhel, A.S.; Xi, Z. Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 2011, 109, E23–E31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.-S.; Webster, J.A.; Madzokere, E.T.; Stephenson, E.B.; Herrero, L.J. Mosquito antiviral defense mechanisms: A delicate balance between innate immunity and persistent viral infection. Parasites Vectors 2019, 12, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larrosa, P.N.F.; Croci, D.O.; Riva, D.A.; Bibini, M.; Luzzi, R.; Saracco, M.; Mersich, S.E.; Rabinovich, G.A.; Peralta, L.M. Apoptosis resistance in HIV-1 persistently-infected cells is independent of active viral replication and involves modulation of the apoptotic mitochondrial pathway. Retrovirology 2008, 5, 19. [Google Scholar] [CrossRef] [Green Version]
- Poenisch, M.; Burger, N.; Staeheli, P.; Bauer, G.; Schneider, U. Protein X of Borna Disease Virus Inhibits Apoptosis and Promotes Viral Persistence in the Central Nervous Systems of Newborn-Infected Rats. J. Virol. 2009, 83, 4297–4307. [Google Scholar] [CrossRef] [Green Version]
- Thepparit, C.; Khakpoor, A.; Khongwichit, S.; Wikan, N.; Fongsaran, C.; Chingsuwanrote, P.; Panraksa, P.; Smith, D.R. Dengue 2 infection of HepG2 liver cells results in endoplasmic reticulum stress and induction of multiple pathways of cell death. BMC Res. Notes 2013, 6, 372. [Google Scholar] [CrossRef] [Green Version]
- Storniolo, A.; Alfano, V.; Carbotta, S.; Ferretti, E.; Di Renzo, L. IRE1α deficiency promotes tumor cell death and eIF2α degradation through PERK dipendent autophagy. Cell Death Discov. 2018, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.-N.; Chen, T.-H.; Chiang, Y.-H.; Peng, J.-Y.; Yang, T.-H.; Cheng, C.-C.; Sofiyatun, E.; Chiu, C.-H.; Chiang-Ni, C.; Chen, W.-J. PERK Signal-Modulated Protein Translation Promotes the Survivability of Dengue 2 Virus-Infected Mosquito Cells and Extends Viral Replication. Viruses 2017, 9, 262. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.I.; Hwang, J.M.; Im, J.H.; Lee, Y.I.; Kim, N.S.; Kim, D.G.; Yu, D.Y.; Moon, H.B.; Park, S.K. Human Hepatitis B Virus-X Protein Alters Mitochondrial Function and Physiology in Human Liver Cells. J. Biol. Chem. 2004, 279, 15460–15471. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Jiang, X.; Chen, X.; Zhu, L.; Zhang, G. The Differential Expression of Mitochondrial Function-Associated Proteins and Antioxidant Enzymes during Bovine Herpesvirus 1 Infection: A Potential Mechanism for Virus Infection-Induced Oxidative Mitochondrial Dysfunction. Mediat. Inflamm. 2019, 2019, 7072917. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Schulze, K.E.; Ghildyal, R.; Henstridge, D.C.; Kolanowski, J.L.; New, E.; Hong, Y.; Hsu, A.C.; Hansbro, P.; Wark, P.; et al. Respiratory syncytial virus co-opts host mitochondrial function to favour infectious virus production. eLife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Bogoyevitch, M.A.; Jans, D.A. Subversion of Host Cell Mitochondria by RSV to Favor Virus Production is Dependent on Inhibition of Mitochondrial Complex I and ROS Generation. Cells 2019, 8, 1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pando-Robles, V.; Batista, C.V. Aedes-Borne Virus–Mosquito Interactions: Mass Spectrometry Strategies and Findings. Vector-Borne Zoonotic Dis. 2017, 17, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Pando-Robles, V.; Oses-Prieto, J.A.; Rodríguez-Gandarilla, M.; Meneses-Romero, E.; Burlingame, A.L.; Batista, C.V. Quantitative proteomic analysis of Huh-7 cells infected with Dengue virus by label-free LC–MS. J. Proteom. 2014, 111, 16–29. [Google Scholar] [CrossRef] [Green Version]
- Perera, R.; Riley, C.; Isaac, G.; Hopf-Jannasch, A.S.; Moore, R.J.; Weitz, K.W.; Pasa-Tolic, L.; Metz, T.; Adamec, J.; Kuhn, R.J. Dengue Virus Infection Perturbs Lipid Homeostasis in Infected Mosquito Cells. PLoS Pathog. 2012, 8, e1002584. [Google Scholar] [CrossRef]
- Chotiwan, N.; Andre, B.G.; Sanchez-Vargas, I.; Islam, M.N.; Grabowski, J.M.; Hopf-Jannasch, A.; Gough, E.; Nakayasu, E.; Blair, C.D.; Belisle, J.T.; et al. Dynamic remodeling of lipids coincides with dengue virus replication in the midgut of Aedes aegypti mosquitoes. PLoS Pathog. 2018, 14, e1006853. [Google Scholar] [CrossRef] [Green Version]
- Fontaine, K.A.; Sanchez, E.L.; Camarda, R.; Lagunoff, M. Dengue Virus Induces and Requires Glycolysis for Optimal Replication. J. Virol. 2014, 89, 2358–2366. [Google Scholar] [CrossRef] [Green Version]
- Fongsaran, C.; Phaonakrop, N.; Roytrakul, S.; Thepparit, C.; Kuadkitkan, A.; Smith, D.R. Voltage Dependent Anion Channel Is Redistributed during Japanese Encephalitis Virus Infection of Insect Cells. Sci. World J. 2014, 2014, 976015. [Google Scholar] [CrossRef] [PubMed]
- Jitobaom, K.; Tongluan, N.; Smith, D.R. Involvement of voltage-dependent anion channel (VDAC) in dengue infection. Sci. Rep. 2016, 6, 35753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Chomyn, A.; Chan, D.C. Disruption of Fusion Results in Mitochondrial Heterogeneity and Dysfunction. J. Biol. Chem. 2005, 280, 26185–26192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Liu, Y.; Dorn, G.W. Mitochondrial Fusion is Essential for Organelle Function and Cardiac Homeostasis. Circ. Res. 2011, 109, 1327–1331. [Google Scholar] [CrossRef]
- Tang, S.; Le, P.K.; Tse, S.; Wallace, D.C.; Huang, T. Heterozygous Mutation of Opa1 in Drosophila Shortens Lifespan Mediated through Increased Reactive Oxygen Species Production. PLoS ONE 2009, 4, e4492. [Google Scholar] [CrossRef] [Green Version]
- Demarco, R.S.; Uyemura, B.S.; D’Alterio, C.; Jones, D.L. Mitochondrial fusion regulates lipid homeostasis and stem cell maintenance in the Drosophila testis. Nature 2019, 21, 710–720. [Google Scholar] [CrossRef]
- Gonçalves, R.L.S.; Machado, A.C.L.; Paiva-Silva, G.O.; Sorgine, M.H.F.; Momoli, M.M.; Oliveira, J.H.M.; Vannier-Santos, M.; Galina, A.; Oliveira, P.; Oliveira, M.F. Blood-Feeding Induces Reversible Functional Changes in Flight Muscle Mitochondria of Aedes aegypti Mosquito. PLoS ONE 2009, 4, e7854. [Google Scholar] [CrossRef] [Green Version]







| Compound | Concentration | Purpose |
|---|---|---|
| Digitonine | 25 μg/mL | Cell permeabilization |
| Succinate | 0.5 mM | Substrate Complex II |
| ADP | 2.5 mM | Substrate Complex IV |
| Cytochrome c | 2.2 mM | Mitochondrial electronic transporter between respiratory complexes III and IV |
| Oligomycin | 5 μg/mL | State 4o respiratory activity |
| CCCP | 0.2 mM | Maximal respiratory activity/State 3u |
| Sodium azide | 2 mM | Inhibitor of ATP synthase |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santana-Román, M.E.; Maycotte, P.; Uribe-Carvajal, S.; Uribe-Alvarez, C.; Alvarado-Medina, N.; Khan, M.; Siddiqui, A.; Pando-Robles, V. Monitoring Mitochondrial Function in Aedes albopictus C6/36 Cell Line during Dengue Virus Infection. Insects 2021, 12, 934. https://doi.org/10.3390/insects12100934
Santana-Román ME, Maycotte P, Uribe-Carvajal S, Uribe-Alvarez C, Alvarado-Medina N, Khan M, Siddiqui A, Pando-Robles V. Monitoring Mitochondrial Function in Aedes albopictus C6/36 Cell Line during Dengue Virus Infection. Insects. 2021; 12(10):934. https://doi.org/10.3390/insects12100934
Chicago/Turabian StyleSantana-Román, María E., Paola Maycotte, Salvador Uribe-Carvajal, Cristina Uribe-Alvarez, Nayeli Alvarado-Medina, Mohsin Khan, Aleem Siddiqui, and Victoria Pando-Robles. 2021. "Monitoring Mitochondrial Function in Aedes albopictus C6/36 Cell Line during Dengue Virus Infection" Insects 12, no. 10: 934. https://doi.org/10.3390/insects12100934
APA StyleSantana-Román, M. E., Maycotte, P., Uribe-Carvajal, S., Uribe-Alvarez, C., Alvarado-Medina, N., Khan, M., Siddiqui, A., & Pando-Robles, V. (2021). Monitoring Mitochondrial Function in Aedes albopictus C6/36 Cell Line during Dengue Virus Infection. Insects, 12(10), 934. https://doi.org/10.3390/insects12100934