The Insect Pest Control Laboratory of the Joint FAO/IAEA Programme: Ten Years (2010–2020) of Research and Development, Achievements and Challenges in Support of the Sterile Insect Technique
Abstract
:Simple Summary
Abstract
1. Historical Background
2. The Insect Pest Control Laboratory of the Joint FAO/IAEA Centre of Nuclear Techniques in Food and Agriculture: Mandate and Objectives
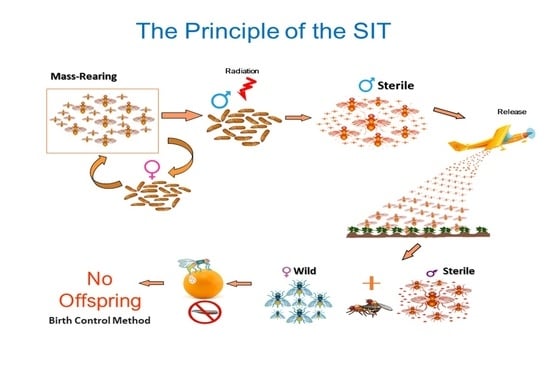
3. Area-Wide Integrated Pest Management and the Sterile Insect Technique
4. Main Research Achievements—Plant Pests
4.1. Genetic Sexing Strains
4.2. Ceratitis Capitata
4.3. Wolbachia-Infected VIENNA-8 Genetic Sexing Strain
4.4. Anastrepha ludens and Anastrepha fraterculus
4.5. White Pupae Bactrocera Introgressed Line
4.6. Cryopreservation
4.7. Species Complexes
4.8. Microbiota and Probiotics
4.9. Tephritid Genomics and Functional Genetics in Support of SIT Applications
4.10. Nutritional, Hormonal and Semio-Chemical Supplements
4.11. Development of the SIT Package for Bactrocera oleae
4.12. Development of the SIT Package for Drosophila suzukii
4.13. Lepidoptera
5. Main Research Achievements—Livestock Pests
5.1. Tsetse Rearing and Handling
5.2. Mating Compatibility and Competitiveness
5.3. Tsetse Symbionts
5.4. Tsetse Symbionts and Irradiation
5.5. Symbionts, Cuticular Hydrocarbons and Mating Choice
5.6. Spiroplasma
5.7. Salivary Gland Hypertrophy Virus (SGHV)
5.8. Classification of the Salivary Gland Hypertrophy Virus
5.9. Antiviral Drugs
5.10. Clean Feeding Protocol
5.11. Tsetse Genomics and Wolbachia Infections
5.12. Sterile Male Quality and Irradiation
5.13. Research in Direct Support of Tsetse AW-IPM Programs
6. Main Research Achievements—Human Disease Vectors
6.1. Combining SIT and IIT for Mosquito Population Suppression
6.2. Aedes aegypti Genetic Sexing Strains
6.3. Anopheles arabiensis Genetic Sexing Strain ANO IPCL1
6.4. Rearing Mosquito Larvae
6.5. Rearing Mosquito Adults
6.6. Irradiation
6.7. Mosquito Symbionts
6.8. Detection of Viruses in Mosquitoes
6.9. Mating Behavior
6.10. Quality Control
6.11. Research in Direct Support of Mosquito AW-IPM Programs
7. Technology Transfer
8. Coordinated Research Projects
9. Challenges and Future Prospects
9.1. Challenges
9.2. Future Research Activities
- Develop SIT package for other suitable insect species
- Develop more cost-efficient mass-rearing techniques
- Establish efficient systems of colony management
- Use of endosymbionts as probiotics to improve the rearing of key insect pests and to enhance mating performance of the sterile males
- Use of semiochemicals and juvenile hormones to enhance mating performance of fruit fly species
- Fine tuning the radiation biology of different species—effects of different ionizing radiation, radiation dose rate, hypoxia, dose fractionating etc.
- Develop improved and more cost-efficient insect release systems using aircraft, gyrocopters or drones
- Unravel the relationships between different endosymbionts and pathogens to enhance male performance, or develop sterile males (in the case of tsetse flies) that are refractory to transmitting pathogens
- Develop more efficient genetic sexing systems, especially for human disease vectors
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vreysen, M.J.B.; Robinson, A.S. Ionising radiation and area-wide management of insect pests to promote sustainable agriculture. A review. Agron. Sustain. Dev. 2011, 31, 233–250. [Google Scholar] [CrossRef] [Green Version]
- FAO. The State of Food Security and Nutrition in the World; FAO: Rome, Italy, 2018. [Google Scholar]
- Roser, M.; Ritchi, H. “Technological Progress” Published online at OurWorldInData.org. Available online: https://ourworldindata.org/technological-progress (accessed on 13 April 2021).
- Dagen, M. History of malaria and its treatment. In Antimalarial Agents; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–48. ISBN 978-0-08-101210-9. [Google Scholar]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Pimentel, D. Area-wide pest management: Environmental, economic, and food issues. In Area-Wide Control of Insect Pests: From Research to Field Implementation; Vreysen, M.J.B., Robinson, A.S., Hendrichs, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 35–47. [Google Scholar]
- Dyck, V.A.; Hendrichs, J.; Robinson, A.S. (Eds.) Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2021; ISBN 978-1-00-303557-2. [Google Scholar]
- Lamm, C.G. The History of the Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture and Its Allied Laboratory (1964–1994); International Atomic Enegy Agency (IAEA): Vienna, Austria, 1994. [Google Scholar]
- Knipling, E.F. Sterile-male method of population control. Science 1959, 130, 902–904. [Google Scholar] [CrossRef]
- Klassen, W.; Vreysen, M.J.B. Area-wide integrated pest management and the sterile insect technique. In The Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 75–112. ISBN 978-1-00-303557-2. [Google Scholar]
- Knipling, E.F. Entomology and the management of man’s environment. Aust. J. Entomol. 1972, 11, 153–167. [Google Scholar] [CrossRef]
- Knipling, E.F. Possibilities of insect control or eradication through the use of sexually sterile males. J. Econ. Entomol. 1955, 48, 459–469. [Google Scholar] [CrossRef]
- Courshee, R. Some aspects of the application of insecticides. Annu. Rev. Entomol. 1960, 5, 327–352. [Google Scholar] [CrossRef]
- Jordan, A.M. Trypanosomiasis Control and African Rural Development; Longman: London, UK, 1986; ISBN 0-582-46356-4. [Google Scholar]
- Kovaleski, A.; Mumford, J. Pulling out the evil by the root: The codling moth Cydia pomonella eradication programme in Brazil. In Area Wide Control of Insect Pests; Vreysen, M.J.B., Robinson, A.S., Hendrichs, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 581–590. ISBN 1-4020-6058-0. [Google Scholar]
- Ford, J.; Nash, T.; Welch, J. Control by Clearing of Vegetation; Afr. Trypanos. Allen Unwin Ltd.: London, UK, 1970; pp. 543–556. [Google Scholar]
- Knipling, E.F. The Basic Principles of Insect Population Suppression and Management; United States Department of Agriculture: Washington, DC, USA, 1979.
- Pereira, R.; Enkerlin, W.; Cáceres, C.; Lu, D.; Vreysen, M.J. Area-wide management of fruit flies using the sterile insect technique. IOBC-WPRS Bull. 2019, 146, 75–78. [Google Scholar]
- Vreysen, M.J. Principles of area-wide integrated tsetse fly control using the sterile insect technique. Med. Trop. Rev. Corps Sante Colon. 2001, 61, 397–411. [Google Scholar]
- Dame, D.A. Control by sterilization of Glossina. In The African Trypanosomiases; Mulligan, H.W., Ed.; CABI: London, UK, 1970; pp. 533–542. [Google Scholar]
- Nagel, P.; Peveling, R. Environment and the Sterile Insect Technique. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Rendon, P.; McInnis, D.; Lance, D.; Stewart, J. Medfly (Diptera: Tephritidae) Genetic sexing: Large-scale field comparison of males-only and bisexual sterile fly releases in Guatemala. J. Econ. Entomol. 2004, 97, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- Franz, G.; Bourtzis, K.; Caceres, C. Practical and operational genetic sexing systems based on classical genetic appraoches in fruit flies, an example for other species amenable to large-scale rearing for the sterile insect technique. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management, 2nd ed.; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 575–604. [Google Scholar]
- Zacharopoulou, A.; Augustinos, A.A.; Drosopoulou, E.; Tsoumani, K.T.; Gariou-Papalexiou, A.; Franz, G.; Mathiopoulos, K.D.; Bourtzis, K.; Mavragani-Tsipidou, P. A review of more than 30 years of cytogenetic studies of Tephritidae in support of sterile insect technique and global trade. Entomol. Exp. Appl. 2017, 164, 204–225. [Google Scholar] [CrossRef] [Green Version]
- Fisher, K. Genetic sexing strains of mediterranean fruit fly (Diptera: Tephritidae); optimizing high temperature treatment of mass-reared temperature-sensitive lethal strains. J. Econ. Entomol. 1998, 91, 1406–1414. [Google Scholar] [CrossRef]
- Augustinos, A.A.; Targovska, E.; Cancio-Martinez, E.; Schorn, G.; Franz, G.; Caceres, C.; Zacharopoulou, A.; Bourtzis, K. Ceratitis Capitata genetic sexing strains: Laboratory evaluation of strains from mass rearing facilities worldwide. Entomol. Exp. Appl. 2017, 164, 305–317. [Google Scholar] [CrossRef] [Green Version]
- Zacharopoulou, A.; Franz, G. Genetic and cytogenetic characterization of genetic sexing strains of Bactrocera Dorsalis and Bactrocera Cucurbitae (Diptera: Tephritidae). J. Econ. Entomol. 2013, 106, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Zepeda-Cisneros, C.S.; Hernández, J.S.M.; Garcáa-Martánez, V.; Ibañez-Palacios, J.; Zacharopoulou, A.; Franz, G. Development, genetic and cytogenetic analyses of genetic sexing strains of the mexican fruit fly, Anastrepha Ludens Loew (Diptera: Tephritidae). BMC Genet. 2014, 15, S1. [Google Scholar] [CrossRef] [Green Version]
- Gariou-Papalexiou, A.; Giardini, M.C.; Augustinos, A.A.; Drosopoulou, E.; Lanzavecchia, S.B.; Cladera, J.L.; Caceres, C.; Bourtzis, K.; Mavragani-Tsipidou, P.; Zacharopoulou, A. Cytogenetic analysis of the south american fruit fly Anastrepha Fraterculus (Diptera:Tephritidae) species complex: Construction of detailed photographic polytene chromosome maps of the Argentinian Af. Sp.1 member. PLoS ONE 2016, 11, e0157192. [Google Scholar] [CrossRef] [PubMed]
- Meza, J.S.; ul Haq, I.; Vreysen, M.J.; Bourtzis, K.; Kyritsis, G.A.; Cáceres, C. Comparison of Classical and transgenic genetic sexing strains of mediterranean fruit fly (Diptera: Tephritidae) for application of the sterile insect technique. PLoS ONE 2018, 13, e0208880. [Google Scholar] [CrossRef] [Green Version]
- Caceres, C. Mass rearing of temperature sensitive genetic sexing strains in the mediterranean fruit fly (Ceratitis Capitata). Genetica 2002, 116, 107–116. [Google Scholar] [CrossRef]
- Porras, M.F.; Meza, J.S.; Rajotte, E.G.; Bourtzis, K.; Cáceres, C. Improving the phenotypic properties of the Ceratitis Capitata (Diptera: Tephritidae) temperature-sensitive lethal genetic sexing strain in support of sterile insect technique applications. J. Econ. Entomol. 2020, 113, 2688–2694. [Google Scholar] [CrossRef]
- Ogaugwu, C.E.; Schetelig, M.F.; Wimmer, E.A. Transgenic sexing system for Ceratitis Capitata (Diptera: Tephritidae) based on female-specific embryonic lethality. Insect Biochem. Mol. Biol. 2013, 43, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rempoulakis, P.; Taret, G.; Haq, I.U.; Wornoayporn, V.; Ahmed, S.; Tomas, U.S.; Dammalage, T.; Gembinsky, K.; Franz, G.; Caceres, C.; et al. Evaluation of quality production parameters and mating behavior of novel genetic sexing strains of the mediterranean fruit fly Ceratitis Capitata (Wiedemann) (Diptera: Tephritidae). PLoS ONE 2016, 11, e0157679. [Google Scholar] [CrossRef]
- Zabalou, S.; Riegler, M.; Theodorakopoulou, M.; Stauffer, C.; Savakis, C.; Bourtzis, K. Wolbachia-induced cytoplasmic incompatibility as a means for insect pest population control. Proc. Natl. Acad. Sci. USA 2004, 101, 15042–15045. [Google Scholar] [CrossRef] [Green Version]
- Kyritsis, G.A.; Augustinos, A.A.; Livadaras, I.; Cáceres, C.; Bourtzis, K.; Papadopoulos, N.T. Medfly-Wolbachia symbiosis: Genotype x genotype interactions determine host’s life history traits under mass rearing conditions. BMC Biotechnol. 2019, 19, 96. [Google Scholar] [CrossRef] [Green Version]
- Reyes Flores, J.; Santiago, M.G.; Hernandez, M.P. The Mexican Fruit Fly Eradication Programme, Proceedings of the Joint International Conference on Area-Wide Control of Insect Pests, Vienna, Austria, 28 May–2 June 1998 and the Fifth International Symposium on Fruit Flies of Economic Importance, Penang, Malaysia, 1–5 June 2008; Tan, K.H., Ed.; Penerbit Universiti Sains Malaysia: Penang, Malaysia, 1998; pp. 377–380. ISBN 983-861-195-6. [Google Scholar]
- Rössler, Y. Automated sexing of Ceratitis Capitata [Dip.: Tephritidae]: The development of strains with inherited sex-limited pupal color dimorphism. Entomophaga 1979, 24, 411–416. [Google Scholar] [CrossRef]
- Meza, J.S.; Cáceres, C.; Bourtzis, K. Slow larvae mutant and its potential to improve the pupal color-based genetic sexing system in mexican fruit fly, (Diptera: Tephritidae). J. Econ. Entomol. 2019, 112, 1604–1610. [Google Scholar] [CrossRef] [PubMed]
- Meza, J.S.; Bourtzis, K.; Zacharopoulou, A.; Gariou-Papalexiou, A.; Cáceres, C. Development and characterization of a pupal-colour based genetic sexing strain of Anastrepha Fraterculus sp. 1 (Diptera: Tephritidae). BMC Genet. 2020, 21, 134. [Google Scholar] [CrossRef]
- Ward, C.M.; Aumann, R.A.; Whitehead, M.A.; Nikolouli, K.; Leveque, G.; Gouvi, G.; Fung, E.; Reiling, S.J.; Djambazian, H.; Hughes, M.A. White pupae phenotype of tephritids is caused by parallel mutations of a MFS transporter. Nat. Commun. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Augustinos, A.A.; Rajamohan, A.; Kyritsis, G.A.; Zacharopoulou, A.; ul Haq, I.; Targovska, A.; Caceres, C.; Bourtzis, K.; Abd-Alla, A.M.M. Cryopreservation of embryos of the mediterranean fruit fly Ceratitis Capitata Vienna 8 genetic sexing strain. PLoS ONE 2016, 11, e0160232. [Google Scholar] [CrossRef] [PubMed]
- Haq, I.U.; Abd-Alla, A.; Tomas, U.S.; Meza, J.S.; Bourtzis, K.; Cáceres, C. Cryopreservation of the Mediterranean Fruit Fly (Diptera: Tephritidae) VIENNA 8 genetic sexing strain: No effect on large scale production of high quality sterile males for SIT applications. PLoS ONE 2019, 14, e0211259. [Google Scholar] [CrossRef] [PubMed]
- Schutze, M.K.; Mahmood, K.; Pavasovic, A.; Bo, W.; Newman, J.; Clarke, A.R.; Krosch, M.N.; Cameron, S.L. One and the same: Integrative taxonomic evidence that Bactrocera Invadens (Diptera: Tephritidae) is the same species as the oriental fruit fly Bactrocera Dorsalis. Syst. Entomol. 2014, 40, 472–486. [Google Scholar] [CrossRef] [Green Version]
- Augustinos, A.A.; Drosopoulou, E.; Gariou-Papalexiou, A.; Bourtzis, K.; Mavragani-Tsipidou, P.; Zacharopoulou, A. The Bactrocera Dorsalis species complex: Comparative Cytogenetic analysis in support of sterile insect technique applications. BMC Genet. 2014, 15 (Suppl. 2), S16. [Google Scholar] [CrossRef] [Green Version]
- Schutze, M.K.; Dammalage, T.; Jessup, A.; Vreysen, M.J.; Wornoayporn, V.; Clarke, A.R. Effects of laboratory colonization on Bactrocera Dorsalis (Diptera, Tephritidae) mating behaviour: “What a difference a year makes”. ZooKeys 2015, 540, 369–383. [Google Scholar] [CrossRef]
- De Meyer, M.; Clarke, A.; Vera, T.; Hendrichs, J. Resolution of cryptic species complexes of tephritid pests to enhance SIT application and facilitate international trade editorial. ZooKeys 2015, 540, 1–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drosopoulou, E.; Pantelidou, C.; Gariou-Papalexiou, A.; Augustinos, A.A.; Chartomatsidou, T.; Kyritsis, G.A.; Bourtzis, K.; Mavragani-Tsipidou, P.; Zacharopoulou, A. The chromosomes and the mitogenome of Ceratitis Fasciventris (Diptera: Tephritidae): Two genetic approaches towards the ceratitis FAR Species Complex Resolution. Sci. Rep. 2017. [Google Scholar] [CrossRef] [Green Version]
- Vera, M.T.; Caceres, C.; Wornoayporn, V.; Islam, A.; Robinson, A.S.; de la Vega, M.H.; Hendrichs, J.P.; Cayol, J.P. Mating incompatibility among populations of the south american fruit fly Anastrepha Fraterculus (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 2006, 99, 387–397. [Google Scholar] [CrossRef]
- Caceres, C.; Segura, D.F.; Vera, M.T.; Wornoayporn, V.; Cladera, J.L.; Teal, P.; Sapountzis, P.; Bourtzis, K.; Zacharopoulou, A.; Robinson, A.S. Incipient speciation revealed in Anastrepha Fraterculus (Diptera: Tephritidae) by studies on mating compatibility, sex pheomones, hybridization, and cytology. Biol. J. Linn. Soc. 2009, 97, 152–165. [Google Scholar] [CrossRef]
- Conte, C.A.; Segura, D.F.; Milla, F.H.; Augustinos, A.; Cladera, J.L.; Bourtzis, K.; Lanzavecchia, S.B. Wolbachia infection in argentinean populations of Anastrepha Fraterculus sp. 1: Preliminary evidence of sex ratio distortion by one of two strains. BMC Microbiol. 2019, 19, 289. [Google Scholar] [CrossRef] [Green Version]
- Devescovi, F.; Conte, C.A.; Augustinos, A.; Martinez, E.I.C.; Segura, D.F.; Caceres, C.; Lanzavecchia, S.B.; Bourtzis, K. Symbionts do not affect the mating incompatibility between the Brazilian-1 and Peruvian morphotypes of the Anastrepha Fraterculus cryptic species complex. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augustinos, A.; Kyritsis, G.; Caceres, C.; Bourtzis, K. Insect Symbiosis in Support of the Sterile Insect Technique. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management, 2nd ed.; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 605–630. [Google Scholar]
- Doudoumis, V.; Blow, F.; Saridaki, A.; Augustinos, A.A.; Dyer, N.A.; Goodhead, I.B.; Solano, P.; Rayaisse, J.-B.; Takac, P.; Mekonnen, S.; et al. Challenging the Wigglesworthia, Sodalis, Wolbachia symbiosis dogma in tsetse flies: Spiroplasma is present in both laboratory and natural populations. Sci. Rep. 2017, 7, 4699. [Google Scholar] [CrossRef] [Green Version]
- Doudoumis, V.; Augustinos, A.; Saridaki, A.; Parker, A.; Abd-Alla, A.M.M.; Bourtzis, K.; Tsiamis, G. Different laboratory populations similar bacterial profile? The case of Glossina Palpalis Gambiensis. BMC Microbiol. 2018, 18, 148. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, D.; Augustinos, A.; Doudoumis, V.; Bel Mokhtar, N.; Maiga, H.; Tsiamis, G.; Bourtzis, K. Multiple factors determine the structure of bacterial communities associated with Aedes Albopictus under artificial rearing conditions. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Augustinos, A.A.; Kyritsis, G.A.; Papadopoulos, N.T.; Abd-Alla, A.M.M.; Cáceres, C.; Bourtzis, K. Exploitation of the medfly gut microbiota for the enhancement of sterile insect technique: Use of Enterobacter sp. in larval diet-based probiotic applications. PLoS ONE 2015, 10, e0136459. [Google Scholar] [CrossRef] [Green Version]
- Kyritsis, G.A.; Augustinos, A.A.; Caceres, C.; Bourtzis, K. Medfly gut microbiota and enhancement of the sterile insect technique: Similarities and differences of Klebsiella Oxytoca and Enterobacter sp. AA26 probiotics during the larval and adult stages of the VIENNA 8D53+ genetic sexing strain. Front. Microbiol. 2017, 8, 2064. [Google Scholar] [CrossRef]
- Azis, K.; Zerva, I.; Melidis, P.; Caceres, C.; Bourtzis, K.; Ntougias, S. Biochemical and nutritional characterization of the medfly gut symbiont Enterobacter sp. AA26 for its use as probiotics in sterile insect technique applications. BMC Biotechnol. 2019, 19, 90. [Google Scholar] [CrossRef] [Green Version]
- Kyritsis, G.A.; Augustinos, A.A.; Ntougias, S.; Papadopoulos, N.T.; Bourtzis, K.; Cáceres, C. Enterobacter sp. AA26 gut symbiont as a protein source for mediterranean fruit fly mass-rearing and sterile insect technique applications. BMC Microbiol. 2019, 19, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augustinos, A.A.; Tsiamis, G.; Cáceres, C.; Abd-Alla, A.M.; Bourtzis, K. Taxonomy, Diet, and developmental stage contribute to the structuring of gut-associated bacterial communities in tephritid pest species. Front. Microbiol. 2019, 10, 2004. [Google Scholar] [CrossRef] [Green Version]
- Ras, E.; Beukeboom, L.W.; Caceres, C.; Bourtzis, K. Review of the role of gut microbiota in mass rearing of the olive fruit fly, Bactrocera Oleae, and its parasitoids. Entomol. Exp. Appl. 2017, 164, 237–256. [Google Scholar] [CrossRef] [Green Version]
- Koskinioti, P.; Ras, E.; Augustinos, A.A.; Tsiamis, G.; Beukeboom, L.W.; Caceres, C.; Bourtzis, K. The effects of geographic origin and antibiotic treatment on the gut symbiotic communities of Bactrocera Oleae populations. Entomol. Exp. Appl. 2019, 167, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Koskinioti, P.; Ras, E.; Augustinos, A.A.; Beukeboom, L.W.; Mathiopoulos, K.D.; Caceres, C.; Bourtzis, K. The impact of fruit fly gut bacteria on the rearing of the parasitic wasp Diachasmimorpha Longicaudata. Entomol. Exp. Appl. 2020, 168, 541–559. [Google Scholar] [CrossRef]
- Papanicolaou, A.; Schetelig, M.F.; Arensburger, P.; Atkinson, P.W.; Benoit, J.B.; Bourtzis, K.; Castañera, P.; Cavanaugh, J.P.; Chao, H.; Childers, C.; et al. The whole genome sequence of the mediterranean fruit fly, Ceratitis Capitata (Wiedemann), reveals insights into the biology and adaptive evolution of a highly invasive pest species. Genome Biol. 2016, 17, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayega, A.; Djambazian, H.; Tsoumani, K.T.; Gregoriou, M.-E.; Sagri, E.; Drosopoulou, E.; Mavragani-Tsipidou, P.; Giorda, K.; Tsiamis, G.; Bourtzis, K. De Novo assembly of the olive fruit fly (Bactrocera Oleae) genome with linked-reads and long-read technologies minimizes gaps and provides exceptional Y chromosome assembly. BMC Genom. 2020, 21, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Meccariello, A.; Salvemini, M.; Primo, P.; Hall, B.; Koskinioti, P.; Dalíková, M.; Gravina, A.; Gucciardino, M.A.; Forlenza, F.; Gregoriou, M.-E. Maleness-on-the-Y (MoY) Orchestrates male sex determination in major agricultural fruit fly pests. Science 2019, 365, 1457–1460. [Google Scholar] [CrossRef] [PubMed]
- Teal, P.E.A.; Gomez-Simuta, Y.; Dueben, B.D.; Holler, T.C.; Olson, S. Improving the efficacy of the sterile insect technique for fruit flies by incorporation of hormone and dietary supplements into adult holding protocols. In Area-Wide Control of Insect Pests: From Research to Field Implementation; Vreysen, M.J.B., Robinson, A.S., Hendrichs, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 163–173. ISBN 1-4020-6058-0. [Google Scholar]
- Teal, P.E.A.; Gomez-Simuta, Y.; Proveaux, A.T. mating experience and juvenile hormone enhance sexual signaling and mating in male caribbean fruit flies. Proc. Natl. Acad. Sci. USA 2000, 97, 3708–3712. [Google Scholar] [CrossRef]
- Haq, I.; Caceres, C.; Hendrichs, J.; Teal, P.; Wornoayporn, V.; Stauffer, C.; Robinson, A.S. Effects of the Juvenile hormone analogue methoprene and dietary protein on male melon fly Bactrocera Cucurbitae (Diptera: Tephritidae) mating success. J. Insect Physiol. 2010, 56, 1503–1509. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.H.; Nishida, R. Mutual reproductive benefits between a wild orchid, Bulbophyllum Patens, and Bactrocera fruit flies via a floral synomone. J. Chem. Ecol. 2000, 26, 533–546. [Google Scholar] [CrossRef]
- Tan, K.H.; Nishida, R. Synomone or kairomone?-Bulbophyllum Apertum flower releases raspberry ketone to attract Bactrocera fruit flies. J. Chem. Ecol. 2005, 31, 497–507. [Google Scholar]
- Barclay, H.J.; McInnis, D.; Hendrichs, J. Modeling the area-wide integration of male annihilation and the simultaneous release of methyl eugenol-exposed Bactrocera spp. sterile males. Ann. Entomol. Soc. Am. 2014, 107, 97–112. [Google Scholar] [CrossRef] [Green Version]
- Shelly, T.E.; Dewire, A.M. Chemically mediated mating success in male oriental fruit flies (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 1994, 87, 375–382. [Google Scholar] [CrossRef]
- Tan, L.T.; Tan, K.H. Automated tephritid fruit fly semiochemical mass feeding structure: Design, construction and testing. J. Appl. Entomol. 2013, 137, 217–229. [Google Scholar] [CrossRef]
- Haq, I.U.; Vreysen, M.J.B.; Cacéres, C.; Shelly, T.E.; Hendrichs, J. Optimizing methyl-eugenol aromatherapy to maximize posttreatment effects to enhance mating competitiveness of male Bactrocera Carambolae (Diptera: Tephritidae). Insect Sci. 2015, 22, 661–669. [Google Scholar] [CrossRef]
- Sohel, A.; Viwat, W.; Polychronis, R.; Emily, A.F.; Ul Haq, I.; Carlos, C.; Hannes, F.P.; Marc, J.B.V. Hybridization and use of grapes as an oviposition substrate improves the adaptation of olive fly Bactrocera Oleae (Rossi) (Diptera: Tephritidae) to artificial rearing conditions. Int. J. Ind. Entomol. 2014, 29, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Nikolouli, K.; Colinet, H.; Renault, D.; Enriquez, T.; Mouton, L.; Gibert, P.; Sassu, F.; Cáceres, C.; Stauffer, C.; Pereira, R. Sterile insect technique and wolbachia symbiosis as potential tools for the control of the invasive species Drosophila Suzukii. J. Pest Sci. 2018, 91, 489–503. [Google Scholar] [CrossRef] [Green Version]
- Sassù, F.; Nikolouli, K.; Caravantes, S.; Taret, G.; Pereira, R.; Vreysen, M.J.; Stauffer, C.; Cáceres, C. Mass-Rearing of Drosophila Suzukii for sterile insect technique application: Evaluation of two oviposition systems. Insects 2019, 10, 448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sassù, F.; Nikolouli, K.; Pereira, R.; Vreysen, M.J.; Stauffer, C.; Cáceres, C. Irradiation dose response under hypoxia for the application of the sterile insect technique in Drosophila Suzukii. PLoS ONE 2019, 14, e0226582. [Google Scholar] [CrossRef]
- Nikolouli, K.; Sassù, F.; Mouton, L.; Stauffer, C.; Bourtzis, K. Combining sterile and incompatible insect techniques for the population suppression of Drosophila Suzukii. J. Pest Sci. 2020, 93, 647–661. [Google Scholar] [CrossRef] [Green Version]
- Drosopoulou, E.; Gariou-Papalexiou, A.; Karamoustou, E.; Gouvi, G.; Augustinos, A.A.; Bourtzis, K.; Zacharopoulou, A. The chromosomes of Drosophila Suzukii (Diptera: Drosophilidae): Detailed photographic polytene chromosomal maps and in situ hybridization data. Mol. Genet. Genom. 2019, 294, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Taret, G.; Sevilla, M.; Wornoayporn, V.; Islam, A.; Ahmad, S.; Caceres, C.; Robinson, A.S.; Vreysen, M.J.B. Mating compatibility among populations of codling moth Cydia Pomonella Linnaeus (Lepidoptera: Tortricidae) from different geographic origins. J. Appl. Entomol. 2010, 134, 207–215. [Google Scholar] [CrossRef]
- Barclay, H.J. Mathematical models for the use of sterile insects. In The Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 147–174. [Google Scholar]
- Vreysen, M.J.B.; Klassen, W.; Carpenter, J.E. Overview of technological advances toward greater efficiency and efficacy in sterile insect-inherited sterility programs against moth pests. Fla. Entomol. 2016, 99, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Chakroun, S.; Rempoulakis, P.; Lebdi-Grissa, K.; Vreysen, M.J.B. Gamma irradiation of the carob or date moth Ectomyelois Ceratoniae: Dose-response effects on egg hatch, fecundity, and survival. Entomol. Exp. Appl. 2017, 164, 257–268. [Google Scholar] [CrossRef] [Green Version]
- Abd-Alla, A.M.M.; Bergoin, M.; Parker, A.; Maniania, N.K.; Vlak, J.M.; Bourtzis, K.; Boucias, D.G.; Aksoy, S. Improving Sterile Insect Technique (SIT) for Tsetse flies through research on their symbionts and pathogens. J. Invertebr. Pathol. 2013, 112, S2–S10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd-Alla, A.M.M.; Kariithi, H.M.; Mohamed, A.H.; Lapiz, E.; Parker, A.G.; Vreysen, M.J.B. Managing Hytrosavirus infections in Glossina Pallidipes colonies: Feeding regime affects the prevalence of salivary gland hypertrophy syndrome. PLoS ONE 2013, 8, e61875. [Google Scholar] [CrossRef] [Green Version]
- Mutika, G.N.; Kabore, I.; Seck, M.T.; Sall, B.; Bouyer, J.; Parker, A.G.; Vreysen, M.J.B. Mating performance of Glossina Palpalis Gambiensis strains from Burkina Faso, Mali, and Senegal. Entomol. Exp. Appl. 2013, 146, 177–185. [Google Scholar] [CrossRef]
- Abd-Alla, A.M.M.; Parker, A.G.; Vreysen, M.J.B.; Bergoin, M. Tsetse salivary gland hypertrophy virus: Hope or hindrance for Tsetse control? PLoS Negl. Trop. Dis. 2011, 5, e1220. [Google Scholar] [CrossRef]
- Moran, Z.R.; Parker, A.G. Near Infrared imaging as a method of studying Tsetse fly (Diptera: Glossinidae) pupal development. J. Insect Sci. 2016, 16, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briceño, R.D.; Wegrzynek, D.; Chinea-Cano, E.; Eberhard, W.G.; dos Santos Rolo, T. Movements and morphology under sexual selection: Tsetse fly genitalia. Ethol. Ecol. Evol. 2010, 22, 385–391. [Google Scholar] [CrossRef]
- Mutika, G.N.; Kabore, I.; Parker, A.G.; Vreysen, M.J.B. Storage of male Glossina Palpalis Gambiensis pupae at low temperature: Effect on emergence, mating and survival. Parasit. Vectors 2014, 7, 465. [Google Scholar] [CrossRef]
- Mutika, G.N.; Parker, A.G. Tolerance of low temperature and sterilizing irradiation in males of Glossina Pallidipes (Diptera: Glossinidae). J. Insect Sci. 2014, 14, 262. [Google Scholar] [CrossRef] [Green Version]
- Mutika, G.N.; Marin, C.; Parker, A.G.; Vreysen, M.J.B.; Boucias, D.G.; Abd-Alla, A.M.M. Impact of salivary gland hypertrophy virus infection on the mating success of male Glossina Pallidipes: Consequences for the sterile insect technique. PLoS ONE 2012, 7, e42188. [Google Scholar] [CrossRef] [PubMed]
- Mutika, G.N.; Parker, A.G.; Vreysen, M.J.B. Tolerance to a combination of low temperature and sterilizing irradiation in male Glossina Palpalis Gambiensis (Diptera: Glossinidae): Simulated transport and release conditions. J. Insect Sci. 2019, 19, 1. [Google Scholar] [CrossRef]
- Mirieri, C.K.; Mutika, G.N.; Bruno, J.; Seck, M.T.; Sall, B.; Parker, A.G.; van Oers, M.M.; Vreysen, M.J.; Bouyer, J.; Abd-Alla, A.M. A new automated chilled adult release system for the aerial distribution of sterile male Tsetse flies. PLoS ONE 2020, 15, e0232306. [Google Scholar] [CrossRef]
- De Beer, C.J.; Venter, G.J.; Vreysen, M.J.B. Improving the diet for the rearing of Glossina Brevipalpis newstead and Glossina Austeni newstead: Blood source and collection-processing-feeding procedures. PLoS ONE 2016, 11, e0168799. [Google Scholar] [CrossRef]
- Desa, G.; Tsegaye, M.; Lelisa, K.; Argiles, R.; Lema, B.; Mekonnen, S.; Briasco, M.; Parker, D.B.A.G.; Bouyer, J. Optimizing the sex ratio to maximize the yield of sterile males in Tsetse mass-rearing colonies. Acad. J. Entomol. 2018, 11, 59–65. [Google Scholar]
- Scolari, F.; Benoit, J.B.; Michalkova, V.; Aksoy, E.; Takac, P.; Abd-Alla, A.M.M.; Malacrida, A.R.; Aksoy, S.; Attardo, G.M. The spermatophore in Glossina Morsitans Morsitans: Insights into male contributions to reproduction. Sci. Rep. 2016, 6, 20334. [Google Scholar] [CrossRef]
- FAO/IAEA. Insect Pest Control Newsletter No. 94; IAEA: Vienna, Austria, 2020. [Google Scholar]
- Hood-Nowotny, R.; Watzka, M.; Mayr, L.; Mekonnen, S.; Kapitano, B.; Parker, A. Intrinsic and synthetic stable isotope marking of Tsetse flies. J. Insect Sci. 2011, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pagabeleguem, S.; Gimonneau, G.; Seck, M.T.; Vreysen, M.J.B.; Sall, B.; Rayaissé, J.-B.; Sidibé, I.; Bouyer, J.; Ravel, S. A molecular method to discriminate between mass-reared sterile and wild Tsetse flies during eradication programmes that have a sterile insect technique component. PLoS Negl. Trop. Dis. 2016, 10, e0004491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, S.; Haq, I.U.; Cáceres, C.; Sto Tomas, U.; Dammalage, T.; Gembinsky, K.; Paulus, H.; Vreysen, M.J.B.; Rempoulakis, P. One for all: Mating compatibility among various populations of olive fruit fly (Diptera: Tephritidae) for application of the sterile insect technique. PLoS ONE 2018, 13, e0206739. [Google Scholar] [CrossRef]
- Simmons, G.S.; Carpenter, J.E.; Suckling, M.; Addison, M.; Dyck, A.; Vreysen, M.J.B. Improved quality management to enhance the efficacy of the sterile insect technique for lepidopteran pests. J. Appl. Entomol. 2010, 134, 261–273. [Google Scholar] [CrossRef]
- Yamada, H.; Vreysen, M.J.B.; Gilles, J.R.L.; Munhenga, G.; Damiens, D.D. The effects of genetic manipulation, dieldrin treatment and irradiation on the mating competitiveness of male Anopheles Arabiensis in field cages. Malar. J. 2014, 13, 318. [Google Scholar] [CrossRef]
- de Beer, C.J.; Venter, G.J.; Vreysen, M.J.B. Determination of the optimal mating age of colonised Glossina Brevipalpis and Glossina Austeni using walk-in field cages in South Africa. Parasit. Vectors 2015, 8, 467. [Google Scholar] [CrossRef] [Green Version]
- Sow, A.; Sidibe, I.; Bengaly, Z.; Bance, A.Z.; Germain, J.; Sawadogo, G.J.; Solano, P.; Vreysen, M.J.B.; Lancelot, R.; Bouyer, J. Irradiated male Tsetse from a 40-year-old colony are still competitive in a riparian forest in Burkina Faso. PLoS ONE 2012, 7, e37124. [Google Scholar] [CrossRef] [Green Version]
- Bassène, M.D.; Seck, M.T.; Pagabeleguem, S.; Fall, A.G.; Sall, B.; Vreysen, M.J.B.; Gimonneau, G.; Bouyer, J. Competitiveness and survival of two strains of Glossina Palpalis Gambiensis in an urban area of Senegal. PLoS Negl. Trop. Dis. 2017, 11, e0006172. [Google Scholar] [CrossRef]
- Michalkova, V.; Benoit, J.B.; Weiss, B.L.; Attardo, G.M.; Aksoy, S. Vitamin B6 generated by obligate symbionts is critical for maintaining proline homeostasis and fecundity in Tsetse flies. Appl. Environ. Microbiol. 2014, 80, 5844–5853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Brelsfoard, C.; Wu, Y.; Aksoy, S. Intercommunity effects on microbiome and GpSGHV density regulation in Tsetse flies. J. Invertebr. Pathol. 2013, 112, S32–S39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saridaki, A.; Bourtzis, K. Wolbachia: More than just a bug in insects’ genitals. Curr. Opin. Microbiol. 2010, 13, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E.L.; Miller, W.J.; Riegler, M. Arthropods shopping for Wolbachia. In Manipulative Tenants: Bacteria Associated with Arthropods; Zchori-Fein, E., Bourtzis, K., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 149–174. [Google Scholar]
- Aksoy, S. Tsetse-a haven for microorganisms. Parasitol. Today 2000, 16, 114–118. [Google Scholar] [CrossRef]
- Aksoy, S.; Weiss, B.; Attardo, G.M. Paratransgenesis applied for control of tsetse transmitted sleeping sickness. In LLC Landes Bioscience; Serap, A., Ed.; Springer Science-Business Media: New York, NY, USA, 2008; pp. 35–48. Available online: www.spinger.com (accessed on 15 January 2021).
- Kariithi, H.M.; Meki, I.K.; Schneider, D.I.; De Vooght, L.; Khamis, F.M.; Geiger, A.; Demirbaş-Uzel, G.; Vlak, J.M.; Ince Ikbal, A.; Kelm, S.; et al. Enhancing vector refractoriness to trypanosome infection: Achievements, challenges and perspectives. BMC Microbiol. 2018, 18, 179. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, S.; Caccone, A.; Galvani, A.P.; Okedi, L.M. Glossina Fuscipes populations provide insights for human african Trypanosomiasis transmission in Uganda. Trends Parasitol. 2013, 29, 394–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doudoumis, V.; Tsiamis, G.; Wamwiri, F.; Brelsfoard, C.; Alam, U.; Aksoy, E.; Dalaperas, S.; Abd-Alla, A.; Ouma, J.; Takac, P.; et al. Detection and characterization of Wolbachia infections in laboratory and natural populations of different species of Tsetse flies (Genus Glossina). BMC Micobiol. 2012, 12, S3. [Google Scholar] [CrossRef] [Green Version]
- Ouedraogo, G.M.S.; Demirbas-Uzel, G.; Rayaisse, J.-B.; Gimonneau, G.; Traore, A.C.; Avgoustinos, A.; Parker, A.G.; Sidibe, I.; Ouedraogo, A.G.; Traore, A.; et al. Prevalence of Trypanosomes, salivary gland hypertrophy virus and Wolbachia in wild populations of Tsetse flies from West Africa. BMC Microbiol. 2018. [Google Scholar] [CrossRef] [Green Version]
- Schneider, D.I.; Garschall, K.I.; Parker, A.G.; Abd-Alla, A.M.M.; Miller, W.J. Global Wolbachia prevalence, titer fluctuations and their potential of causing cytoplasmic incompatibilities in Tsetse flies and hybrids of Glossina Morsitans subgroup species. J. Invertebr. Pathol. 2013, 112, S104–S115. [Google Scholar] [CrossRef] [Green Version]
- Schneider, D.I.; Parker, A.G.; Abd-Alla, A.M.M.; Miller, W.J. High-sensitivity detection of cryptic Wolbachia in the African Tsetse fly (Glossina spp.). BMC Microbiol. 2018, 18, S1. [Google Scholar] [CrossRef] [PubMed]
- Kariithi, H.M.; Ahmadi, M.; Parker, A.G.; Franz, G.; Ros, V.I.D.; Haq, I.; Elashry, A.M.; Vlak, J.M.; Bergoin, M.; Vreysen, M.J.B.; et al. Prevalence and Genetic variation of salivary gland hypertrophy virus in wild populations of the Tsetse fly Glossina Pallidipes from Southern and Eastern Africa. J. Invertebr. Pathol. 2013, 112, S123–S132. [Google Scholar] [CrossRef] [PubMed]
- Meki, I.K.; Kariithi, H.M.; Ahmadi, M.; Parker, A.G.; Vreysen, M.J.B.; Vlak, J.M.; van Oers, M.M.; Abd-Alla, A.M.M. Hytrosavirus genetic diversity and eco-regional spread in Glossina species. BMC Microbiol. 2018, 18, 143. [Google Scholar] [CrossRef]
- Malele, I.I.; Manangwa, O.; Nyingilili, H.H.; Kiwika, W.A.; Lyaruu, E.A.; Msangi, A.R.; Ouma, J.O.; Nkwengulila, G.; Abd-Alla, A.M.M. Prevalence of SGHV among Tsetse species of economic importance in Tanzania and their implication for SIT application. J. Invertebr. Pathol. 2013, 112, S133–S137. [Google Scholar] [CrossRef]
- Van Den Abbeele, J.; Bourtzis, K.; Weiss, B.; Cordón-Rosales, C.; Miller, W.; Abd-Alla, A.M.M.; Parker, A.G. Enhancing Tsetse fly refractoriness to trypanosome infection-a new IAEA coordinated research project. J. Invertebr. Pathol. 2013, 112, S142–S147. [Google Scholar] [CrossRef] [PubMed]
- Abd-Alla, A.M.M.; Tsiamis, G.; Boucias, D.G. Special issue on enhancing vector refractoriness to trypanosome infection-foreword. BMC Microbiol. 2018, 18, 141. [Google Scholar] [CrossRef] [PubMed]
- De Vooght, L.; Caljon, G.; De Ridder, K.; van den Abbeele, J. Delivery of a functional anti-trypanosome nanobody in different Tsetse fly tissues via a bacterial symbiont, Sodalis Glossinidius. Microb. Cell Fact. 2014, 13, 156. [Google Scholar] [CrossRef] [PubMed]
- Caljon, G.; De Vooght, L.; Van Den Abbeele, J. Options for the delivery of anti-pathogen molecules in arthropod vectors. J. Invertebr. Pathol. 2013, 112, S75–S82. [Google Scholar] [CrossRef]
- De Vooght, L.; Caljon, G.; Stijlemans, B.; de Beatselier, P.; Coosemans, M.; Van Den Abbeele, J. Expression and extracellular release of a functional anti-trypanosome nanobody (R) in Sodalis Glossinidius, a bacterial symbiont of the Tsetse fly. Microb. Cell Fact. 2012, 11, 23. [Google Scholar] [CrossRef] [Green Version]
- De Vooght, L.; Caljon, G.; Van Hees, J.; Van Den Abbeele, J. Paternal transmission of a secondary symbiont during mating in the viviparous Tsetse fly. Mol. Biol. Evol. 2015, 32, 1977–1980. [Google Scholar] [CrossRef] [Green Version]
- Demirbas-Uzel, G.; De Vooght, L.; Parker, A.G.; Vreysen, M.J.B.; Mach, R.L.; Van Den Abbeele, J.; Abd-Alla, A.M.M. Combining paratransgenesis with SIT: Impact of ionizing radiation on the DNA copy number of Sodalis Glossinidius in Tsetse flies. BMC Microbiol. 2018, 18, 160. [Google Scholar] [CrossRef] [Green Version]
- Brucker, R.M.; Bordenstein, S.R. Speciation by symbiosis. Trends Ecol. Evol. 2012, 27, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Engl, T.; Michalkova, V.; Weiss, B.L.; Uzel, G.D.; Takac, P.; Miller, W.J.; Abd-Alla, A.M.M.; Aksoy, S.; Kaltenpoth, M. Effect of antibiotic treatment and gamma-irradiation on cuticular hydrocarbon profiles and mate choice in Tsetse flies (Glossina m. Morsitans). BMC Microbiol. 2018, 18, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitnall, A.B.M. The Trypanosome Infections of Glossina pallidipes in the Umfolosi Game Reserve, Zululand; Preliminary Report; Director of Veterinary Services: Onderstepoort, South Africa, 1932; pp. 21–30. [Google Scholar]
- Whitnall, A.B.M. The Trypanosome Infections of Glossina Pallidipes in the Umfolosi Game Reserve, Zululand. Onderstepoort J. Vet. Sci. Anim. Ind. 1934, 2, 7–21. [Google Scholar]
- Burtt, E. Hypertrophied salivary glands in Glossina: Evidence that G. Pallidipes with this abnormality is particularly suited to trypanosome infection. Ann. Trop. Med. Parasitol. 1945, 39, 11–13. [Google Scholar] [CrossRef]
- Jaenson, T.G.T. Virus-like rods associated with salivary gland hyperplasia in Tsetse, Glossina Pallidipes. Trans. R. Soc. Trop. Med. Hyg. 1978, 72, 234–238. [Google Scholar] [CrossRef]
- Jaenson, T.G.T. Reproductive Biology of the Tsetse Glossina Pallidipes Austen (Diptera, Glossinidae) with Special Reference to Mating Behaviour; Acta Universitatis Upsaliensis: Uppsalla, Sweden, 1978; pp. 1–40. [Google Scholar]
- Sang, R.C.; Jura, W.G.Z.O.; Otieno, L.H.; Ogaja, P. Ultrastructural changes in the milk gland of Tsetse Glossina Morsitans Centralis (Diptera; Glissinidae) female infected by a DNA virus. J. Invertebr. Pathol. 1996, 68, 253–259. [Google Scholar] [CrossRef]
- Sang, R.C.; Jura, W.G.Z.O.; Otieno, L.H.; Mwangi, R.W. The effects of a DNA virus infection on the reproductive potential of female Tsetse flies, Glossina Morsitans Centralis and Glossina Morsitans Morsitans (Diptera: Glossinidae). Mem. Inst. Oswaldo Cruz 1998, 93, 861–864. [Google Scholar] [CrossRef]
- Jura, W.G.Z.O.; Zdarek, J.; Otieno, L.H. A Simple method for artificial infection of Tsetse, Glossina Morsitans Morsitans larvae with the DNA virus of G. Pallidipes. Insect Sci. Appl. 1993, 14, 383–387. [Google Scholar] [CrossRef]
- Jura, W.G.Z.O.; Davies-Cole, J.O.A. Some aspects of mating behavior of Glossina Morsitans Morsitans males infected with a DNA virus. Biol. Control 1992, 2, 188–192. [Google Scholar] [CrossRef]
- Jura, W.G.Z.O.; Otieno, L.H.; Chimtawi, M.M.B. Ultrastructural evidence for trans-ovum transmission of the DNA virus of Tsetse, Glossina Pallidipes (Diptera: Glossinidae). Curr. Microbiol. 1989, 18, 1–4. [Google Scholar] [CrossRef]
- Gouteux, J.-P. Prevalence of enlarged salivary glands in Glossina Palpalis, G. Pallicera, and G. Nigrofusca (Diptera: Glossinidae) from the Vavoua area, Ivory Coast. J. Med. Entomol. 1987, 24, 268. [Google Scholar] [CrossRef]
- Vreysen, M.J.B.; Saleh, K.M.; Ali, M.Y.; Abdulla, A.M.; Zhu, Z.-R.; Juma, K.G.; Dyck, V.A.; Msangi, A.R.; Mkonyi, P.A.; Feldmann, H.U. Glossina Austeni (Diptera: Glossinidae) eradicated on the Island of Unguja, Zanzibar, using the sterile insect technique. J. Econ. Entomol. 2000, 93, 123–135. [Google Scholar] [CrossRef]
- Abd-Alla, A.M.M.; Kariithi, H.M.; Parker, A.G.; Robinson, A.S.; Kiflom, M.; Bergoin, M.; Vreysen, M.J.B. Dynamics of the salivary gland hypertrophy virus in laboratory colonies of Glossina Pallidipes (Diptera: Glossinidae). Virus Res. 2010, 150, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Demirbas-Uzel, G.; Kariithi, H.M.; Parker, A.G.; Vreysen, M.J.B.; Mach, R.L.; Abd-Alla, A.M.M. Susceptibility of Tsetse species to Glossina Pallidipes salivary gland hypertrophy virus (GpSGHV). Front. Microbiol. 2018, 9, 701. [Google Scholar] [CrossRef] [Green Version]
- Demirbas-Uzel, G.; Parker, A.G.; Vreysen, M.J.; Mach, R.L.; Bouyer, J.; Takac, P.; Abd-Alla, A.M. Impact of Glossina Pallidipes salivary gland hypertrophy virus (GpSGHV) on a heterologous Tsetse fly host, Glossina Fuscipes Fuscipes. BMC Microbiol. 2018, 18, 161. [Google Scholar] [CrossRef] [Green Version]
- Abd-Alla, A.M.M.; Cousserans, F.; Parker, A.G.; Jehle, J.A.; Parker, N.J.; Vlak, J.M.; Robinson, A.S.; Bergoin, M. Genome analysis of a Glossina Pallidipes salivary gland hypertrophy virus (GpSGHV) reveals a Novel large double-stranded circular DNA virus. J. Virol. 2008, 82, 4595–4611. [Google Scholar] [CrossRef] [Green Version]
- Abd-Alla, A.M.; Kariithi, H.M.; Cousserans, F.; Parker, N.J.; Ince, I.A.; Scully, E.D.; Boeren, S.; Geib, S.M.; Mekonnen, S.; Vlak, J.M.; et al. Comprehensive annotation of the Glossina Pallidipes salivary gland hypertrophy virus from ethiopian Tsetse flies: A proteogenomics approach. J. Gen. Virol. 2016, 97, 1010–1031. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Maruniak, A.; Abd-Alla, A.M.M.; Salem, T.Z.; Parker, A.G.; van Oers, M.M.; Maruniak, J.E.; Kim, W.; Burand, J.P.; Cousserans, F.; Robinson, A.S.; et al. Two viruses that cause salivary gland hypertrophy in Glossina Pallidipes and Musca Domestica are related and form a distinct phylogenetic clade. J. Gen. Virol. 2009, 90, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Kariithi, H.M.; Vlak, J.M.; Jehle, J.A.; Bergoin, M.; Boucias, D.G.; Abd-Alla, A.M.M. Ictv Report Consortium, null ICTV virus taxonomy profile: Hytrosaviridae. J. Gen. Virol. 2019, 100, 1271–1272. [Google Scholar] [CrossRef]
- Jehle, J.A.; Abd-Alla, A.M.M.; Wang, Y. Phylogeny and evolution of Hytrosaviridae. J. Invertebr. Pathol. 2013, 112, S62–S67. [Google Scholar] [CrossRef]
- Abd-Alla, A.M.M.; Boucias, D.G.; Bergoin, M. Hytrosaviruses: Structure and Genomic Properties; Asgari, S., Johnson, K.N., Eds.; Caister Academic Press: Norfolk, UK, 2010; pp. 103–121. ISBN 978-1-9044557-71-4. [Google Scholar]
- Abd-Alla, A.M.M.; Vlak, J.M.; Bergoin, M.; Maruniak, J.E.; Parker, A.G.; Burand, J.P.; Jehle, J.A.; Boucias, D.G. Hytrosaviridae: A proposal for classification and nomenclature of a new insect virus family. Arch. Virol. 2009, 154, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Lietze, V.U.; Abd-Alla, A.M.M.; Vreysen, M.J.B.; Geden, C.J.; Boucias, D.G. Salivary gland hypertrophy viruses: A novel group of insect pathogenic viruses. Annu. Rev. Entomol. 2010, 56, 63–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kariithi, H.M.; van Lent, J.W.; Boeren, S.; Abd-Alla, A.M.M.; Ince, I.A.; van Oers, M.M.; Vlak, J.M. Correlation between structure, protein composition, morphogenesis and cytopathology of Glossina Pallidipes salivary gland hypertrophy virus. J. Gen. Virol. 2013, 94, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Orlov, I.; Drillien, R.; Spehner, D.; Bergoin, M.; Abd-Alla, A.M.; Klaholz, B.P. Structural features of the salivary gland hypertrophy virus of the Tsetse Fly revealed by cryo-electron microscopy and tomography. Virology 2018, 514, 165–169. [Google Scholar] [CrossRef]
- Kariithi, H.M.; van Oers, M.M.; Vlak, J.M.; Vreysen, M.J.B.; Parker, A.G.; Abd-Alla, A.M.M. Virology, epidemiology and pathology of Glossina hytrosavirus, and its control prospects in laboratory colonies of the Tsetse fly, Glossina Pallidipes (Diptera; Glossinidae). Insects 2013, 4, 287–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kariithi, H.M. Glossina hytrosavirus Control Strategies in Tsetse Fly Factories: Application of Infectomics in Virus Management; Wageningen University: Wageningen, The Netherlands, 2013. [Google Scholar]
- Kariithi, H.M.; Ince, A.I.; Boeren, S.; Vervoort, J.; Bergoin, M.; van Oers, M.M.; Abd-Alla, A.M.M.; Vlak, J.M. Proteomic analysis of Glossina Pallidipes salivary gland hypertrophy virus virions for immune intervention in Tsetse fly colonies. J. Gen. Virol. 2010, 91, 3065–3074. [Google Scholar] [CrossRef] [PubMed]
- Kariithi, H.M.; Ince, I.A.; Boeren, S.; Abd-Alla, A.M.M.; Parker, A.G.; Aksoy, S.; Vlak, J.M.; van Oers, M.M. The salivary secretome of the Tsetse fly Glossina Pallidipes (Diptera: Glossinidae) infected by salivary gland hypertrophy virus. PLoS Negl. Trop. Dis. 2011, 5, e1371. [Google Scholar] [CrossRef] [Green Version]
- Kariithi, H.M.; Ince, I.A.; Boeren, S.; Murungi, E.K.; Meki, I.K.; Otieno, E.A.; Nyanjom, S.R.G.; van Oers, M.M.; Vlak, J.M.; Abd-Alla, A.M.M. Comparative analysis of salivary gland proteomes of two Glossina species that exhibit differential hytrosavirus pathologies. Front. Microbiol. 2016, 7, 89. [Google Scholar] [CrossRef]
- Kariithi, H.M.; Boeren, S.; Murungi, E.K.; Vlak, J.M.; Abd-Alla, A.M.M. A proteomics approach reveals molecular manipulators of distinct cellular processes in the salivary glands of Glossina m. Morsitans in response to Trypanosoma b. Brucei infections. Parasit. Vectors 2016, 9, 424. [Google Scholar] [CrossRef] [Green Version]
- Boucias, D.G.; Kariithi, H.M.; Bourtzis, K.; Schneider, D.I.; Kelley, K.; Miller, W.J.; Parker, A.G.; Abd-Alla, A.M.M. Transgenerational transmission of the Glossina Pallidipes hytrosavirus depends on the presence of a functional symbiome. PLoS ONE 2013, 8, e61150. [Google Scholar] [CrossRef] [PubMed]
- Meki, I.K.; İnce, İ.A.; Kariithi, H.M.; Boucias, D.G.; Orhan Ozcan, O.; Parker, A.G.; Vlak, J.M.; Oers, M.M.V.; Abdalla, A.M.M. Expression profile of Glossina Pallidipes microRNAs during symptomatic and asymptomatic infection with Glossina Pallidipes salivary gland hypertrophy virus (Hytrosavirus). Front. Microbiol. 2018, 9, 2037. [Google Scholar] [CrossRef]
- Meki, I.K.; Kariithi, H.M.; Parker, A.G.; Vreysen, M.J.; Ros, V.I.; Vlak, J.M.; van Oers, M.M.; Abd-Alla, A.M. RNA Interference-based antiviral immune response against the salivary gland hypertrophy virus in Glossina Pallidipes. BMC Microbiol. 2018, 18, 170. [Google Scholar] [CrossRef] [PubMed]
- Kariithi, H.M.; Boucias, D.G.; Murung, E.K.; Meki, I.K.; Demirbas-Uzel, G.; van Oers, M.M.; Vreysen, M.J.B.; Abd-Alla, A.M.M. Coevolution of Hytrosaviruses and host immune responses. BMC Microbiol. 2018. [Google Scholar] [CrossRef]
- Abd-Alla, A.M.M.; Adun, H.; Parker, A.G.; Vreysen, M.J.B.; Bergoin, M. The antiviral drug valacyclovir successfully suppresses salivary gland hypertrophy virus (SGHV) in laboratory colonies of Glossina Pallidipes. PLoS ONE 2012, 7, e38417. [Google Scholar] [CrossRef]
- Abd-Alla, A.M.M.; Marin, C.; Parker, A.; Vreysen, M. Antiviral drug valacyclovir treatment combined with a clean feeding system enhances the suppression of salivary gland hypertrophy in laboratory colonies of Glossina Pallidipes. Parasit. Vectors 2014, 7, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kariithi, H.M.; Meki, I.K.; Boucias, D.G.; Abd-Alla, A.M. Hytrosaviruses: Current status and perspective. Curr. Opin. Insect Sci. 2017, 22, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Abd-Alla, A.M.; Meki, I.K.; Demirbas-Uzel, G. Insect viruses as biocontrol agents: Challenges and opportunities. In Cottage Industry of Biocontrol Agents and Their Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 277–295. [Google Scholar]
- International Glossina genome initiative genome sequence of the Tsetse fly (Glossina Morsitans): Vector of african Trypanosomiasis. Science 2014, 344, 380–386. [CrossRef] [Green Version]
- Brelsfoard, C.; Tsiamis, G.; Falchetto, M.; Gomulski, L.M.; Telleria, E.; Alam, U.; Doudoumis, V.; Scolari, F.; Benoit, J.B.; Swain, M.; et al. Presence of extensive Wolbachia symbiont insertions discovered in the genome of its host Glossina Morsitans Morsitans. PLoS Negl. Trop. Dis. 2014, 8, e2728. [Google Scholar] [CrossRef] [Green Version]
- Attardo, G.M.; Abd-Alla, A.M.M.; Acosta-Serrano, A.; Allen, J.E.; Bateta, R.; Benoit, J.B.; Bourtzis, K.; Caers, J.; Caljon, G.; Christensen, M.B.; et al. Comparative genomic analysis of six Glossina genomes, vectors of african Trypanosomes. Genome Biol. 2019, 20, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augustinos, A.A.; Meki, I.K.; Demirbas-Uzel, G.; Ouédraogo, G.M.S.; Saridaki, A.; Tsiamis, G.; Parker, A.G.; Abd-Alla, A.M.M.; Bourtzis, K. Nuclear and Wolbachia-based multimarker approach for the rapid and accurate identification of Tsetse species. BMC Microbiol. 2018, 18, 147. [Google Scholar] [CrossRef] [Green Version]
- Vreysen, M.J.B.; Gerardo-Abaya, J.; Cayol, J.P. Lessons from area-wide integrated pest management (AW-IPM) programmes with an SIT component: An FAO/IAEA perspective. In Area-Wide Control of Insect Pests: From Research to Field Implementation; Vreysen, M.J.B., Robinson, A.S., Hendrichs, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 723–744. [Google Scholar]
- Vreysen, M.J.B.; Saleh, K.M.; Lancelot, R.; Bouyer, J. Factory Tsetse flies must behave like wild flies: A prerequisite for the sterile insect technique. PLoS Negl. Trop. Dis. 2011, 5, e907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seck, M.T.; Pagabeleguem, S.; Bassene, M.D.; Fall, A.G.; Diouf, T.A.R.; Sall, B.; Vreysen, M.J.B.; Rayaissé, J.B.; Takac, P.; Sidibé, I.; et al. Quality of sterile male Tsetse after long distance transport as chilled, irradiated pupae. PLoS Negl. Trop. Dis. 2015, 9, e0004229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diallo, S.; Seck, M.T.; Rayaissé, J.B.; Fall, A.G.; Bassene, M.D.; Sall, B.; Sanon, A.; Vreysen, M.J.B.; Takac, P.; Parker, A.G.; et al. Chilling, irradiation and transport of male Glossina palpalis gambiensis pupae: Effect on the emergence, flight ability and survival. PLoS ONE 2019, 14, e0216802. [Google Scholar] [CrossRef] [Green Version]
- de Beer, C.J.; Moyaba, P.; Boikanyo, S.N.B.; Majatladi, D.; Yamada, H.; Venter, G.J.; Vreysen, M.J.B. Evaluation of radiation sensitivity and mating performance of Glossina Brevipalpis males. PLoS Negl. Trop. Dis. 2017, 11, e0005473. [Google Scholar] [CrossRef] [PubMed]
- De Beer, C.J.; Moyaba, P.; Boikanyo, S.N.; Majatladi, D.; Venter, G.J.; Vreysen, M.J. Gamma irradiation and male Glossina Austeni mating performance. Insects 2020, 11, 522. [Google Scholar] [CrossRef]
- Bouyer, J.; Seck, M.T.; Sall, B.; Ndiaye, E.Y.; Guerrini, L.; Vreysen, M.J.B. Stratified entomological sampling in preparation for an area-wide integrated pest management program: The example of Glossina Palpalis Gambiensis (Diptera: Glossinidae) in the niayes of Senegal. J. Med. Entomol. 2010, 47, 543–552. [Google Scholar] [CrossRef]
- Seck, M.T.; Bouyer, J.; Sall, B.; Bengaly, Z.; Vreysen, M.J.B. The Prevalence of african animal Trypanosomoses and Tsetse presence in western Senegal. Parasite 2010, 17, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Solano, P.; Kaba, D.; Ravel, S.; Dyer, N.A.; Sall, B.; Vreysen, M.J.B.; Seck, M.T.; Darbyshir, H.; Gardes, L.; Donnelly, M.J.; et al. Population genetics as a tool to select Tsetse Control strategies: Suppression or eradication of Glossina Palpalis Gambiensis in the niayes of Senegal. PLoS Negl. Trop. Dis. 2010, 4, e692. [Google Scholar] [CrossRef] [Green Version]
- Vreysen, M.; Seck, M.; Sall, B.; Mbaye, A.; Bassène, M.; Fall, A.; Lo, M.; Bouyer, J. Area-wide integrated management of a Glossina Palpalis Gambiensis population from the niayes area of Senegal: A review of operational research in support of a phased conditional approach. In Area-Wide Integrated Pest Management: Development and Field Application; Hendrichs, J., Pereira, R., Vreysen, M.J., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 275–303. [Google Scholar]
- Dicko, A.H.; Lancelot, R.; Seck, M.T.; Guerrini, L.; Sall, B.; Lo, M.; Vreysen, M.J.B.; Lefrançãois, T.; Fonta, W.M.; Peck, S.L.; et al. Using species distribution models to optimize vector control in the framework of the Tsetse eradication campaign in Senegal. Proc. Natl. Acad. Sci. USA 2014, 111, 10149–10154. [Google Scholar] [CrossRef] [Green Version]
- Bouyer, F.; Seck, M.T.; Dicko, A.H.; Sall, B.; Lo, M.; Vreysen, M.J.B.; Chia, E.; Bouyer, J.; Wane, A. Ex-ante benefit-cost analysis of the elimination of a Glossina Palpalis Gambiensis population in the niayes of Senegal. PLoS Negl. Trop. Dis. 2014, 8, e3112. [Google Scholar] [CrossRef] [Green Version]
- Ciss, M.; Bassène, M.D.; Seck, M.T.; Mbaye, A.G.; Sall, B.; Fall, A.G.; Vreysen, M.J.; Bouyer, J. Environmental impact of Tsetse eradication in Senegal. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Pagabeleguem, S.; Seck, M.T.; Sall, B.; Vreysen, M.J.B.; Gimonneau, G.; Fall, A.G.; Bassene, M.; Sidibé, I.; Rayaissé, J.B.; Belem, A.M.G.; et al. Long distance transport of irradiated male Glossina Palpalis Gambiensis Pupae and its impact on sterile male yield. Parasit. Vectors 2015, 8, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagabeleguem, S.; Ravel, S.; Dicko, A.H.; Vreysen, M.J.; Parker, A.; Takac, P.; Huber, K.; Sidibé, I.; Gimonneau, G.; Bouyer, J. Influence of temperature and relative humidity on survival and fecundity of three Tsetse strains. Parasit. Vectors 2016, 9, 520. [Google Scholar] [CrossRef] [PubMed]
- Bouyer, J.; Ravel, S.; Dujardin, J.P.; De Meeus, T.; Vial, L.; Thevenon, S.; Guerrini, L.; Sidibe, I.; Solano, P. Population structuring of Glossina Palpalis Gambiensis (Diptera: Glossinidae) according to landscape fragmentation in the Mouhoun River, Burkina Faso. J. Med. Entomol. 2007, 44, 788–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koné, N.; Bouyer, J.; Ravel, S.; Vreysen, M.J.B.; Domagni, K.T.; Causse, S.; Solano, P.; De Meeus, T. Contrasting population structures of two vectors of african Trypanosomoses in Burkina Faso: Consequences for control. PLoS Negl. Trop. Dis. 2011, 5, e1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vreysen, M.J.B.; Balenghien, T.; Saleh, K.; Maiga, S.; Koudougou, Z.; Cecchi, G.; Bouyer, J. Release-recapture studies confirm dispersal of Glossina Palpalis Gambiensis between River Basins in Mali. PLoS Negl. Trop. Dis. 2013, 7, e2022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Beer, C.J.; Venter, G.J.; Kappmeier Green, K.; Esterhuizen, J.; de Klerk, D.G.; Ntshangase, J.; Vreysen, M.J.B.; Pienaar, R.; Motloang, M.; Ntandiso, L.; et al. An update of the Tsetse fly (Diptera: Glossinidae) distribution and african animal Trypanosomosis prevalence in North-Eastern KwaZulu-Natal, South Africa. Onderstepoort J. Vet. Res. 2016, 83, a1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Beer, C.J.; Venter, G.J.; Vreysen, M.J.B.; Mulandane, F.C.; Neves, L.; Mdluli, S.; Koekemoer, O. Using genetic and phenetic markers to assess population isolation within the southernmost Tsetse fly belt in Africa. Onderstepoort J. Vet. Res. 2019, 86, e1–e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cecchi, G.; Paone, M.; Argilés Herrero, R.; Vreysen, M.J.B.; Mattioli, R.C. Developing a continental atlas of the distribution and trypanosomal infection of Tsetse Flies (Glossina species). Parasit. Vectors 2015, 8, 284. [Google Scholar] [CrossRef] [Green Version]
- Barclay, H.J.; Vreysen, M.J.B. A Dynamic population model for Tsetse (Diptera: Glossinidae) area-wide integrated pest management. Popul. Ecol. 2010, 53, 89–110. [Google Scholar] [CrossRef]
- Barclay, H.J.; Vreysen, M.J.B. Conclusions from a dynamic population model for Tsetse: Response to comments. Popul. Ecol. 2011, 53, 417–420. [Google Scholar] [CrossRef]
- Bouyer, J.; Lancelota, R. Using genetic data to improve species distribution models. Infect. Genet. Evol. 2017, 63, 292–294. [Google Scholar] [CrossRef]
- Bouyer, J.; Dicko, A.H.; Cecchi, G.; Ravel, S.; Guerrini, L.; Solano, P.; Vreysen, M.J.; De Mees, T.; Lancelot, R. Mapping landscape friction to locate isolated tsetse populations that are candidates for elimination. Proc. Natl. Acad. Sci. USA 2015, 112, 14575–14580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chikowore, G.; Dicko, A.H.; Chinwada, P.; Zimba, M.; Shereni, W.; Roger, F.; Bouyer, J.; Guerrini, L. A pilot study to delimit Tsetse target populations in Zimbabwe. PLoS Negl. Trop. Dis. 2017, 11, e0005566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Meeûs, T.; Ravel, S.; Solano, P.; Bouyer, J. Negative density-dependent dispersal in Tsetse flies: A risk for control campaigns? Trends Parasitol. 2019, 35, 615–621. [Google Scholar] [CrossRef]
- Barclay, H.J.; Vreysen, M.J.B. The interaction of dispersal and control methods for the riverine Tsetse fly Glossina Palpalis Gambiensis (Diptera: Glossinidae): A modelling study. Popul. Ecol. 2013, 55, 53–68. [Google Scholar] [CrossRef]
- Dicko, A.H.; Percoma, L.; Sow, A.; Adam, Y.; Mahama, C.; Sidibé, I.; Dayo, G.-K.; Thévenon, S.; Fonta, W.; Sanfo, S.; et al. A spatio-temporal model of african animal trypanosomosis risk. PLoS Negl. Trop. Dis. 2015, 9, e0003921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Percoma, L.; Sow, A.; Pagabeleguem, S.; Dicko, A.H.; Serdebéogo, O.; Ouédraogo, M.; Rayaissé, J.-B.; Bouyer, J.; Belem, A.M.G.; Sidibé, I. Impact of an integrated control campaign on Tsetse populations in Burkina Faso. Parasit. Vectors 2018, 11, 270. [Google Scholar] [CrossRef]
- Gilles, J.R.L.; Schetelig, M.F.; Scolari, F.; Marec, F.; Capurro, M.L.; Franz, G.; Bourtzis, K. Towards mosquito sterile insect technique programmes: Exploring genetic, molecular, mechanical and behavioural methods of sex separation in mosquitoes. Acta Trop. 2014, 132S, S178–S187. [Google Scholar] [CrossRef]
- Papathanos, P.A.; Bourtzis, K.; Tripet, F.; Bossin, H.; Virginio, J.F.; Capurro, M.L.; Pedrosa, M.C.; Guindo, A.; Sylla, L.; Coulibaly, M.B.; et al. A perspective on the need and current status of efficient sex separation methods for mosquito genetic control. Parasit. Vectors 2018, 11, 654. [Google Scholar] [CrossRef]
- Lutrat, C.; Giesbrecht, D.; Marois, E.; Whyard, S.; Baldet, T.; Bouyer, J. Sex sorting for pest control: It’s raining men! Trends Parasitol. 2019, 35, 649–662. [Google Scholar] [CrossRef] [Green Version]
- Lees, R.S.; Gilles, J.R.L.; Hendrichs, J.; Vreysen, M.J.B.; Bourtzis, K. Back to the future: The sterile insect technique against mosquito disease vectors. Curr. Opin. Insect Sci. 2015, 10, 156–162. [Google Scholar] [CrossRef] [Green Version]
- Bourtzis, K.; Lees, R.S.; Hendrichs, J.; Vreysen, M.J.B. More than one rabbit out of the hat: Radiation, transgenic and symbiont-based approaches for sustainable management of mosquito and Tsetse fly populations. Acta Trop. 2016, 157, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zheng, X.; Xi, Z.; Bourtzis, K.; Gilles, J.R.L. Combining the sterile insect technique with the incompatible insect technique: I-Impact of Wolbachia infection on the fitness of triple-and double-infected strains of Aedes Albopictus. PLoS ONE 2015, 10, e0121126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Lees, R.S.; Xi, Z.; Gilles, J.R.L.; Bourtzis, K. Combining the sterile insect technique with Wolbachia-based approaches: II-A safer approach to Aedes Albopictus Population suppression programmes, designed to minimize the consequences of inadvertent female release. PLoS ONE 2015, 10, e0135194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lees, R.S.; Xi, Z.; Bourtzis, K.; Gilles, J.R.L. Combining the sterile insect technique with the incompatible insect technique: III-robust mating competitiveness of irradiated triple Wolbachia-infected Aedes Albopictus males under semi-field conditions. PLoS ONE 2016, 11, e0151864. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, D.; Li, Y.; Yang, C.; Wu, Y.; Liang, X.; Liang, Y.; Pan, X.; Hu, L.; Sun, Q.; et al. Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature 2019, 572, 56–61. [Google Scholar] [CrossRef]
- Carvalho, D.O.; Torres-Monzon, J.A.; Koskinioti, P.; Dilrukshi Wijegunawardana, N.A.; Liang, X.; Pillwax, G.; Xi, Z.; Bourtzis, K. Aedes Aegypti lines for combined sterile insect technique and incompatible insect technique applications: The importance of host genomic background. Entomol. Exp. Appl. 2020, 168, 560–572. [Google Scholar] [CrossRef]
- Koskinioti, P.; Augustinos, A.A.; Carvalho, D.O.; Misbah-ul-Haq, M.; Pillwax, G.; de la Fuente, L.D.; Salvador-Herranz, G.; Herrero, R.A.; Bourtzis, K. Genetic Sexing strains for the population suppression of the mosquito vector Aedes Aegypti. Philos. Trans. R. Soc. B 2021, 376, 20190808. [Google Scholar] [CrossRef] [PubMed]
- Augustinos, A.A.; Misbah-ul-Haq, M.; Carvalho, D.O.; de la Fuente, L.D.; Koskinioti, P.; Bourtzis, K. Irradiation induced inversions suppress recombination between the M Locus and morphological markers in Aedes Aegypti. BMC Genet. 2020, 21, 142. [Google Scholar] [CrossRef] [PubMed]
- Gunathilaka, N.; Ranathunge, T.; Udayanga, L.; Wijegunawardena, A.; Gilles, J.R.L.; Abeyewickreme, W. Use of mechanical and behavioural methods to eliminate female Aedes Aegypti and Aedes Albopictus for sterile insect technique and incompatible insect technique applications. Parasit. Vectors 2019, 12, 1–14. [Google Scholar] [CrossRef]
- Mamai, W.; Maiga, H.; Somda, N.S.B.; Wallner, T.; Konczal, A.; Yamada, H.; Bouyer, J. Aedes Aegypti larval development and pupal production in the FAO/IAEA mass-rearing rack and factors influencing sex sorting efficiency. Parasite 2020, 27, 43. [Google Scholar] [CrossRef]
- Yamada, H.; Benedict, M.Q.; Malcolm, C.A.; Oliva, C.F.; Soliban, S.M.; Gilles, J.R.L. Genetic sex separation of the malaria vector, Anopheles Arabiensis, by exposing eggs to dieldrin. Malar. J. 2012, 11, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliva, C.F.; Benedict, M.Q.; Soliban, S.M.; Lemperiere, G.; Balestrino, F.; Gilles, J.R. Comparisons of life-history characteristics of a genetic sexing strain with laboratory strains of Anopheles Arabiensis (Diptera: Culicidae) from Northern Sudan. J. Med. Entomol. 2012, 49, 1045–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, H.; Vreysen, M.J.B.; Bourtzis, K.; Tschirk, W.; Chadee, D.D.; Gilles, J.R.L. The Anopheles Arabiensis genetic sexing strain ANO IPCL1 and its application potential for the sterile insect technique in integrated vector management programmes. Acta Trop. 2015, 142, 138–144. [Google Scholar] [CrossRef]
- Yamada, H.; Jandric, Z.; Chhem-Kieth, S.; Vreysen, M.J.B.; Rathor, M.N.; Gilles, J.R.L.; Cannavan, A. Anopheles Arabiensis egg treatment with dieldrin for sex separation leaves residues in male adult mosquitoes that can bioaccumulate in goldfish (Carassius Auratus Auratus). Environ. Toxicol. Chem. 2013, 32, 2786–2791. [Google Scholar] [CrossRef] [Green Version]
- Ndo, C.; Poumachu, Y.; Metitsi, D.; Awono-Ambene, H.P.; Tchuinkam, T.; Gilles, J.L.R.; Bourtzis, K. Isolation and characterization of a temperature-sensitive lethal strain of Anopheles Arabiensis for SIT-based application. Parasit. Vectors 2018, 11, 659. [Google Scholar] [CrossRef] [Green Version]
- Lowe, R.E.; Fowler, J.E.F.; Bailey, D.L.; Dame, D.A.; Savage, K.E. Separation of sexes of adult Anopheles Albimanus by feeding of insecticide-laden blood. Mosq. News 1981, 41, 634–638. [Google Scholar]
- Yamada, H.; Soliban, S.; Vreysen, M.J.B.; Chadee, D.D.; Gilles, J.R.L. Eliminating female Anopheles Arabiensis by spiking blood meals with toxicants as a sex separation method in the context of the sterile insect technique. Parasit. Vectors 2013, 6, 197. [Google Scholar] [CrossRef] [Green Version]
- Oliva, C.F.; Benedict, M.Q.; Lemperiere, G.; Gilles, J. Laboratory selection for an accelerated mosquito sexual development rate. Malar. J. 2011, 10, 135. [Google Scholar] [CrossRef]
- Zheng, M.L.; Zhang, D.J.; Damiens, D.D.; Yamada, H.; Gilles, J.R.L. Standard operating procedures for standardized mass rearing of the dengue and chikungunya vectors Aedes Aegypti and Aedes Albopictus (Diptera: Culicidae)-I-egg quantification. Parasit. Vectors 2015, 8, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, M.L.; Zhang, D.J.; Damiens, D.D.; Lees, R.S.; Gilles, J.R. Standard operating procedures for standardized mass rearing of the dengue and chikungunya vectors Aedes Aegypti and Aedes Albopictus (Diptera: Culicidae)-II-egg storage and hatching. Parasit. Vectors 2015, 8, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maïga, H.; Damiens, D.; Diabaté, A.; Dabiré, R.K.; Ouédraogo, G.A.; Lees, R.S.; Gilles, J.R.L. Large-scale Anopheles Arabiensis egg quantification methods for mass-rearing operations. Malar. J. 2016, 15, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO/IAEA. Guidelines for Mass Rearing Aedes Mosquitoes. Version 1.0; IAEA: Vienna, Austria, 2020. [Google Scholar]
- Khan, I.; Damiens, D.; Soliban, S.M.; Gilles, J.R.L. Effects of drying eggs and egg storage on hatchability and development of Anopheles Arabiensis. Malar. J. 2013, 12, 318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobb, L.; Munhenga, G.; Yamada, H.; Koekemoer, L. The effect of egg storage of laboratory reared Anopheles Arabiensis (Diptera: Culicidae) on egg hatch synchronisation, pupation success and pupal production time. Afr. Entomol. 2019, 27, 360–365. [Google Scholar] [CrossRef]
- Yamada, H.; Kraupa, C.; Lienhard, C.; Parker, A.G.; Maiga, H.; de Oliveira Carvalho, D.; Zheng, M.; Wallner, T.; Bouyer, J. Mosquito mass rearing: Who’s eating the eggs? Parasite 2019, 26, 75. [Google Scholar] [CrossRef]
- Damiens, D.; Benedict, M.Q.; Wille, M.; Gilles, J.R.L. An Inexpensive and effective larval diet for Anopheles Arabiensis (Diptera: Culicidae): Eat like a horse, a bird or a fish? J. Med. Entomol. 2012, 49, 1001–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puggioli, A.; Balestrino, F.; Damiens, D.; Lees, R.S.; Soliban, S.M.; Madakacherry, O.; Dindo, M.L.; Bellini, R.; Gilles, J.R.L. Efficiency of three diets for larval development in mass rearing Aedes Albopictus (Diptera: Culicidae). J. Med. Entomol. 2013, 50, 819–825. [Google Scholar] [CrossRef] [Green Version]
- Gilles, J.R.L.; Lees, R.S.; Soliban, S.M.; Benedict, M.Q. Density-Dependent Effects in esperimental larval populations of Anopheles Arabiensis (Diptera: Culicidae) can be negative, neutral, or overcompensatory depending on density and diet levels. J. Med. Entomol. 2011, 48, 296–304. [Google Scholar] [CrossRef] [Green Version]
- Bimbilé Somda, N.S.; Dabiré, K.R.; Maiga, H.; Yamada, H.; Mamai, W.; Gnankiné, O.; Diabaté, A.; Sanon, A.; Bouyer, J.; Gilles, J.L. Cost-effective larval diet mixtures for mass rearing of Anopheles Arabiensis patton (Diptera: Culicidae). Parasit. Vectors 2017, 10, 619. [Google Scholar] [CrossRef] [Green Version]
- Yahouédo, G.A.; Djogbénou, L.; Saïzonou, J.; Assogba, B.S.; Makoutodé, M.; Gilles, J.R.L.; Maïga, H.; Mouline, K.; Soukou, B.K.; Simard, F. Effect of three larval diets on larval development and male sexual performance of Anopheles Gambiae s.s. Acta Trop. 2014, 132S, S96–S101. [Google Scholar] [CrossRef]
- Epopa, P.S.; Maiga, H.; de Sales Hien, D.F.; Dabire, R.K.; Lees, R.S.; Giles, J.; Tripet, F.; Baldet, T.; Damiens, D.; Diabate, A. Assessment of the developmental success of anopheles coluzzii larvae under different nutrient regimes: Effects of diet quality, food amount and larval density. Malar. J. 2018, 17, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Mamai, W.; Lobb, L.N.; Bimbilé Somda, N.S.; Maiga, H.; Yamada, H.; Lees, R.S.; Bouyer, J.; Gilles, J.R. Optimization of mass-rearing methods for Anopheles Arabiensis larval stages: Effects of Rearing water temperature and larval density on mosquito life-history traits. J. Econ. Entomol. 2018, 111, 2383–2390. [Google Scholar] [CrossRef]
- Somda, N.S.B.; Maïga, H.; Mamai, W.; Yamada, H.; Ali, A.; Konczal, A.; Gnankiné, O.; Diabaté, A.; Sanon, A.; Dabiré, K.R. Insects to feed insects-feeding aedes mosquitoes with flies for laboratory rearing. Sci. Rep. 2019, 9, 1–13. [Google Scholar]
- Mamai, W.; Somda, N.S.B.; Maiga, H.; Konczal, A.; Wallner, T.; Bakhoum, M.T.; Yamada, H.; Bouyer, J. Black soldier fly (Hermetia Illucens) larvae powder as a larval diet ingredient for mass-rearing aedes mosquitoes. Parasite 2019, 26, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bond, J.; Ramírez-Osorio, A.; Marina, C.; Fernández-Salas, I.; Liedo, P.; Dor, A.; Williams, T. Efficiency of two larval diets for mass-rearing of the mosquito Aedes Aegypti. PLoS ONE 2017, 12, e0187420. [Google Scholar] [CrossRef] [Green Version]
- Hood-Nowotny, R.; Schwarzinger, B.; Schwarzinger, C.; Soliban, S.; Madakacherry, O.; Aigner, M.; Watzka, M.; Gilles, J. An analysis of diet quality, how it controls fatty acid profiles, isotope signatures and stoichiometry in the malaria mosquito Anopheles Arabiensis. PLoS ONE 2012, 7, e45222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO/IAEA. Guidelines for Standardised Mass Rearing of Anopheles Mosquitoes-Version 1.0; FAO: Rome, Italy, 2017; p. 44. [Google Scholar]
- Balestrino, F.; Benedict, M.Q.; Gilles, J.R. A new larval tray and rack system for improved mosquito mass rearing. J. Med. Entomol. 2012, 49, 595–605. [Google Scholar] [CrossRef]
- Balestrino, F.; Puggioli, A.; Gilles, J.R.L.; Bellini, R. Validation of a new larval rearing unit for Aedes Albopictus (Diptera: Culicidae) Mass Rearing. PLoS ONE 2014, 9, e91914. [Google Scholar] [CrossRef] [Green Version]
- Soma, D.D.; Maiga, H.; Mamai, W.; Bimbile-Somda, N.S.; Venter, N.; Ali, A.B.; Yamada, H.; Diabate, A.; Fournet, F.; Ouedraogo, G.A.; et al. Does mosquito mass-rearing produce an inferior mosquito? Malar. J. 2017. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Zhang, M.; Wu, Y.; Gilles, J.R.L.; Yamada, H.; Wu, Z.; Xi, Z.; Zheng, X. Establishment of a medium-scale mosquito facility: Optimization of the larval mass-rearing unit for Aedes Albopictus (Diptera: Culicidae). Parasit. Vectors 2017, 10, 569. [Google Scholar] [CrossRef]
- Dogan, M.; Gunay, F.; Puggioli, A.; Balestrino, F.; Oncu, C.; Alten, B.; Bellini, R. Establishment of a satellite rearing facility to support the release of sterile Aedes Albopictus males. I. Optimization of mass rearing parameters. Acta Trop. 2016, 159, 62–68. [Google Scholar] [CrossRef]
- Mamai, W.; Maiga, H.; Bimbilé Somda, N.S.; Wallner, T.; Masso, O.B.; Resch, C.; Yamada, H.; Bouyer, J. Does tap water quality compromise the production of Aedes mosquitoes in genetic control projects? Insects 2021, 12, 57. [Google Scholar] [CrossRef] [PubMed]
- Mamai, W.; Lees, R.S.; Maiga, H.; Gilles, J.R.L. Reusing larval rearing water and its effect on development and quality of Anopheles Arabiensis Mosquitoes. Malar. J. 2016, 15, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamai, W.; Hood-Nowotny, R.; Maiga, H.; Ali, A.B.; Bimbile-Somda, N.S.; Soma, D.D.; Yamada, H.; Lees, R.S.; Gilles, J.R. Reverse osmosis and ultrafiltration for recovery and reuse of larval rearing water in Anopheles Arabiensis mass production: Effect of water quality on larval development and fitness of emerging adults. Acta Trop. 2017, 170, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Mamai, W.; Maiga, H.; Gárdos, M.; Bán, P.; Bimbilé Somda, N.S.; Konczal, A.; Wallner, T.; Parker, A.; Balestrino, F.; Yamada, H.; et al. The efficiency of a new automated mosquito larval counter and its impact on larval survival. Sci. Rep. 2019, 9, 7413. [Google Scholar] [CrossRef] [PubMed]
- Balestrino, F.; Gilles, J.R.L.; Soliban, S.M.; Nirschl, A.; Benedict, Q.E.; Benedict, M.Q. Mosquito mass rearing technology: A cold-water vortex device for continuous unattended separation of Anopheles Arabiensis pupae from larvae. J. Am. Mosq. Control Assoc. 2011, 27, 227–235. [Google Scholar] [CrossRef]
- Damiens, D.; Soliban, S.M.; Balestrino, F.; Alsir, R.; Vreysen, M.J.B.; Gilles, J.R.L. Different blood and sugar feeding regimes affect the productivity of Anopheles Arabiensis colonies (Diptera: Culicidae). J. Med. Entomol. 2013, 50, 336–343. [Google Scholar] [CrossRef]
- Balestrino, F.; Soliban, S.M.; Gilles, J.; Oliva, C.; Benedict, M.Q. Ovipositional behavior in the context of mass rearing of Anopheles Arabiensis. J. Am. Mosq. Control Assoc. 2010, 26, 365–372. [Google Scholar] [CrossRef]
- Balestrino, F.; Puggioli, A.; Bellini, R.; Petric, D.; Gilles, J.R.L. Mass production cage for Aedes Albopictus (Diptera: Culicidae). J. Med. Entomol. 2014, 51, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Maiga, H.; Bimbilé-Somda, N.S.; Yamada, H.; Wood, O.; Damiens, D.; Mamai, W.; Balestrino, F.; Lees, R.S.; Dabiré, R.K.; Diabaté, A.; et al. Enhancements to the mass-rearing cage for the malaria vector, Anopheles Arabiensis for improved adult longevity and egg production. Entomol. Exp. Appl. 2017, 164, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Mamai, W.; Bimbile-Somda, N.S.; Maiga, H.; Juarez, J.G.; Muosa, Z.A.I.; Ali, A.B.; Lees, R.S.; Gilles, J.R.L. Optimization of mosquito egg production under mass rearing setting: Effects of cage volume, blood meal source and adult population density for the malaria vector, Anopheles Arabiensis. Malar. J. 2017, 17, 377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maïga, H.; Mamai, W.; Somda, N.S.B.; Konczal, A.; Wallner, T.; Herranz, G.S.; Herrero, R.A.; Yamada, H.; Bouyer, J. Reducing the cost and assessing the performance of a novel adult mass-rearing cage for the Dengue, Chikungunya, Yellow Fever and Zika vector, Aedes Aegypti (Linnaeus). PLoS Negl. Trop. Dis. 2019, 13, e0007775. [Google Scholar] [CrossRef]
- Maïga, H.; Mamai, W.; Bimbilé Somda, N.S.; Wallner, T.; Poda, B.S.; Salvador-Herranz, G.; Argiles-Herrero, R.; Yamada, H.; Bouyer, J. Assessment of a novel adult mass-rearing cage for Aedes Albopictus (Skuse) and Anopheles Arabiensis (Patton). Insects 2020, 11, 801. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, Y.; Sun, Q.; Zheng, X.; Gilles, J.R.; Yamada, H.; Wu, Z.; Xi, Z.; Wu, Y. Establishment of a medium-scale mosquito facility: Tests on mass production cages for Aedes Albopictus (Diptera: Culicidae). Parasit. Vectors 2018, 11, 189. [Google Scholar] [CrossRef] [PubMed]
- LaChance, L.E. The induction of dominant lethal mutations in insects by ionizing radiation and chemicals-as related to the sterile male technique of insect control. In Genetic Basis of the Sterile Insect Technique; Wright, J.W., Pal, R., Eds.; Elsevier: Amsterdam, The Netherlands, 1967; pp. 617–650. [Google Scholar]
- Curtis, C.F. Induced sterility in insects. Adv. Reprod. Physiol. 1971, 5, 120–165. [Google Scholar]
- Helinski, M.E.H.; Parker, A.G.; Knols, B.G. Radiation-Induced sterility for pupal and adult stages of the malaria mosquito Anopheles Arabiensis. Malar. J. 2006, 5, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Malek, A.A.; Wakid, A.M.; Tantawy, A.O.; El Gazzar, L.M. Studies on Factors Influencing the Induction of Sterility in Anopheles Pharoensis Theobald by Gamma Radiation; No. CONF-740379-4; Nehme, M., Hassan, A., Eds.; National Council for Scientific Research: Beirut, Lebanon, 1975; pp. 161–174. [Google Scholar]
- Ali, S.R.; Rozeboom, L.E. Observations on sterilization of Anopheles (C.) Albimanus Wiedemann by x-irradiation. Mosq. News 1972, 32, 574–579. [Google Scholar]
- Khan, G.Z.; Salman, M.; Khan, I.; Zeb, A.; Shah, J.A.; Hussain, A.; Akbar, I.; Bibi, A.; Anwar, S.; Badshah, T. Assessment of irradiation doses for sterility of vector mosquito and subsequent mating compatibility with wild females. J. Entomol. Zool. Stud. 2015, 3, 138–141. [Google Scholar]
- Bellini, R.; Balestrino, F.; Medici, A.; Gentile, G.; Veronesi, R.; Carrieri, M. Mating Competitiveness of Aedes Albopictus radio-sterilized males in large enclosures exposed to natural conditions. J. Med. Entomol. 2013, 50, 94–102. [Google Scholar] [CrossRef] [Green Version]
- Ernawan, B.; Sasmita, H.; Parikesit, A. Sterility of male Aedes Aegypti post γ-ray sterilization. JAPS J. Anim. Plant Sci. 2018, 28, 973–977. [Google Scholar]
- Helinski, M.E.H.; Knols, B.G.J. Mating Competitiveness of Male Anopheles Arabiensis Mosquitoes irradiated with a partially or fully sterilizing dose in small and large laboratory cages. J. Med. Entomol. 2008, 45, 698–705. [Google Scholar] [CrossRef]
- Lebon, C.; Soupapoule, K.; Wilkinson, D.A.; Goff, G.L.; Damiens, D.; Gouagna, L.C. Laboratory evaluation of the effects of sterilizing doses of γ-Rays from Caesium-137 source on the daily flight activity and flight performance of Aedes Albopictus males. PLoS ONE 2018, 13, e0202236. [Google Scholar] [CrossRef] [PubMed]
- Maïga, H.; Damiens, D.; Niang, A.; Sawadogo, S.P.; Fatherhaman, O.; Lees, R.S.; Roux, O.; Dabiré, K.R.; Ouédraogo, G.A.; Tripet, F.; et al. Mating Competitiveness of sterile male Anopheles Coluzzii in large cages. Malar. J. 2014, 13, 460. [Google Scholar] [CrossRef] [Green Version]
- Munhenga, G.; Brooke, B.D.; Gilles, J.R.; Slabbert, K.; Kemp, A.; Dandalo, L.C.; Wood, O.R.; Lobb, L.N.; Govender, D.; Renke, M. Mating competitiveness of sterile genetic sexing strain males (GAMA) under laboratory and semi-field conditions: Steps towards the use of the sterile insect technique to control the major malaria Vector Anopheles Arabiensis in South Africa. Parasit. Vectors 2016, 9, 122. [Google Scholar] [CrossRef] [Green Version]
- Yamada, H.; Maiga, H.; Juarez, J.; De Oliveira Carvalho, D.; Mamai, W.; Ali, A.; Bimbile-Somda, N.S.; Parker, A.G.; Zhang, D.; Bouyer, J. Identification of critical factors that significantly affect the dose-response in mosquitoes irradiated as pupae. Parasit. Vectors 2019, 12, 435. [Google Scholar] [CrossRef]
- Helinski, M.E.H.; Parker, A.G.; Knols, B.G.J. Radiation biology of mosquitoes. Malar. J. 2009, 8, S6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, H.; Parker, A.G.; Oliva, C.F.; Balestrino, F.; Gilles, J.R.L. X-ray-induced sterility in Aedes Albopictus and male longevity following irradiation. J. Med. Entomol. 2014, 51, 811–816. [Google Scholar] [CrossRef] [Green Version]
- Balestrino, F.; Medici, A.; Candini, G.; Carrieri, M.; Maccagnani, B.; Calvitti, M.; Maini, S.; Bellini, R. Gamma ray dosimetry and mating capacity studies in the laboratory on Aedes Albopictus males. J. Med. Entomol. 2010, 47, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Maiga, H.; Bimbile-Somda, N.S.; Carvalho, D.O.; Mamai, W.; Kraupa, C.; Parker, A.G.; Abrahim, A.; Weltin, G.; Wallner, T. The role of oxygen depletion and subsequent radioprotective effects during irradiation of mosquito pupae in water. Parasit. Vectors 2020, 13, 1–10. [Google Scholar] [CrossRef]
- Bond, J.G.; Osorio, A.R.; Avila, N.; Gómez-Simuta, Y.; Marina, C.F.; Fernández-Salas, I.; Liedo, P.; Dor, A.; Carvalho, D.O.; Bourtzis, K. Optimization of irradiation dose to Aedes Aegypti and Ae. Albopictus in a sterile insect technique program. PLoS ONE 2019, 14, e0212520. [Google Scholar] [CrossRef] [PubMed]
- Ndo, C.; Yamada, H.; Damiens, D.D.; N’do, S.; Seballos, G.; Gilles, J.R. X-ray Sterilization of the An. Arabiensis genetic sexing strain “ANO IPCL1” at pupal and adult stages. Acta Trop. 2013, 131C, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Oliva, C.F.; Maier, M.J.; Gilles, J.; Jacquet, M.; Lemperiere, G.; Quilici, S.; Vreysen, M.J.; Schooneman, F.; Chadee, D.D.; Boyer, S. Effects of irradiation, presence of females, and sugar supply on the longevity of sterile males Aedes Albopictus (Skuse) under semi-field conditions on reunion island. Acta Trop. 2012, 125, 287–293. [Google Scholar] [CrossRef]
- Curtis, C.F. Radiation Sterilization; Ross Institute of Tropical Hygiene: London, UK, 1976; pp. 31–76. [Google Scholar]
- El-Gazzar, L.M.; Dame, D.A.; Smittle, B.J. Fertility and competitiveness of Culex Quinquefasciatus males irradiated in nitrogen. J. Econ. Entomol. 1983, 76, 821–823. [Google Scholar] [CrossRef]
- Hallman, G.J.; Hellmich, R.L. Modified Atmosphere Storage May Reduce Efficacy of Irradiation Phytosanitary Treatments. In Proceedings of the IX International Controlled Atmosphere Research Conference, East Lansing, MI, USA, 5–10 July 2005; pp. 159–162. [Google Scholar]
- Mehta, K.; Parker, A. Characterization and dosimetry of a practical X-ray alternative to self-shielded gamma irradiators. Radiat. Phys. Chem. 2011, 80, 107–113. [Google Scholar] [CrossRef]
- Khoury, H.J.; da Silva, E.J.; Mehta, K.; de Barros, V.S.; Asfora, V.K.; Guzzo, P.L.; Parker, A.G. Alanine-EPR as a transfer standard dosimetry system for low energy X radiation. Radiat. Phys. Chem. 2015, 116, 147–150. [Google Scholar] [CrossRef]
- Balestrino, F.; Mathis, A.; Langs, S.; Veronesi, E. Sterilization of Hulecoeteomyia Japonica Japonica (= Aedes Japonicus Japonicus) (Theobald, 1901) by high-energy photon irradiation: Implications for a sterile insect technique approach in Europe. Med. Vet. Entomol. 2016, 30, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Poda, S.B.; Guissou, E.; Gilles, J.; Rayaisse, J.-B.; Lefèvre, T.; Roux, O.; Dabire, R.K. Impact of irradiation on reproductive performance of wild and laboratory Anopheles Arabiensis mosquitoes. Am. J. Trop. Med. Hyg. 2017, 97, 53. [Google Scholar]
- Ageep, T.B.; Damiens, D.; Alsharif, B.; Ahmed, A.; Salih, E.H.O.; Ahmed, F.T.A.; Diabaté, A.; Lees, R.S.; Gilles, J.R.L.; El Sayed, B.B. Participation of irradiated Anopheles Arabiensis males in swarms following field release in Sudan. Malar. J. 2014, 13, 484. [Google Scholar] [CrossRef] [Green Version]
- Guissou, E.; Poda, S.; de Sales Hien, D.F.; Yerbanga, S.R.; Da, D.F.; Cohuet, A.; Fournet, F.; Roux, O.; Maiga, H.; Diabaté, A. Effect of irradiation on the survival and susceptibility of female Anopheles Arabiensis to natural isolates of plasmodium falciparum. Parasit. Vectors 2020, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Yamada, H.; Kraupa, C.; Canic, S.; Busquets, N.; Talavera, S.; Jiolle, D.; Vreysen, M.J.; Bouyer, J.; Abd-Alla, A.M. High sensitivity of one-step real-time reverse transcription quantitative PCR to Detect low virus titers in large mosquito pools. Parasit. Vectors 2020, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Helinski, M.E.H.; Knols, B.G. Sperm quantity and size variation in un-irradiated and irradiated males of the malaria mosquito Anopheles Arabiensis patton. Acta Trop. 2009, 109, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Ponlawat, A.; Harrington, L.C. Age and body size influence male sperm capacity on the dengue vector Aedes Aegypti (Diptera: Culicidae). J. Med. Entomol. 2007, 44, 422–426. [Google Scholar] [CrossRef]
- Damiens, D.; Vreysen, M.J.B.; Gilles, J.R.L. Anopheles Arabiensis sperm production after genetic manipulation, dieldrin treatment, and irradiation. J. Med. Entomol. 2013, 50, 314–316. [Google Scholar] [CrossRef] [Green Version]
- Patterson, R.S.; Sharma, V.P.; Singh, K.R.P.; LaBrecque, G.C.; Seetharam, P.L.; Grover, K.K. Use of radiosterilized males to control indigenous populations of Culex Pipiens Quinquefasciatus say: Laboratory and field studies. Mosq. News 1975, 35, 1–7. [Google Scholar]
- Bimbilé Somda, N.S.; Poda, B.S.; Sawadogo, P.S.; Gnankiné, O.; Maiga, H.; Fournet, F.; Lees, R.S.; Bouyer, J.; Gilles, J.; Sanon, A. Ecology of reproduction of Anopheles Arabiensis in an urban area of Bobo-Dioulasso, Burkina Faso (West Africa): Monthly swarming and mating frequency and their relation to environmental factors. PLoS ONE 2018, 13, e0205966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawadogo, S.P.; Diabaté, A.; Toé, H.K.; Sanon, A.; Lefevre, T.; Baldet, T.; Gilles, J.; Simard, F.; Gibson, G.; Sinkins, S.; et al. Effects of age and size on Anopheles Gambiae s.s. male mosquito mating success. J. Med. Entomol. 2013, 50, 285–293. [Google Scholar] [CrossRef]
- Maiga, H.; Dabire, R.K.; Lehmann, T.; Tripet, F.; Diabate, A. Variation in energy reserves and role of body size in the mating system of Anopheles Gambiae. J. Vector Ecol. 2012, 37, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Maïga, H.; Niang, A.; Sawadogo, S.; Dabiré, R.K.; Lees, R.S.; Gilles, J.R.L.; Tripet, F.; Diabaté, A. Role of nutritional reserves and body Size in Anopheles Gambiae males mating success. Acta Trop. 2014, 132S, S102–S107. [Google Scholar] [CrossRef] [PubMed]
- Oliva, C.F.; Damiens, D.; Vreysen, M.J.; Lemperière, G.; Gilles, J. Reproductive Ssrategies of Aedes Albopictus (Diptera: Culicidae) and implications for the sterile insect technique. PLoS ONE 2013, 8, e78884. [Google Scholar] [CrossRef] [Green Version]
- Oliva, C.F.; Damiens, D.; Benedict, M.Q. Male reproductive biology of Aedes mosquitoes. Acta Trop. 2014, S132, S12–S19. [Google Scholar] [CrossRef]
- Maïga, H.; Gilles, J.R.; Lees, R.S.; Yamada, H.; Bouyer, J. Demonstration of resistance to satyrization behavior in Aedes Aegypti from La Réunion Island. Parasite 2020, 27, 10. [Google Scholar] [CrossRef] [Green Version]
- Fried, M. Determination of sterile-insect competitiveness. J. Econ. Entomol. 1971, 64, 869–872. [Google Scholar] [CrossRef]
- Bouyer, J.; Vreysen, M.J.B. Yes, Irradiated sterile male mosquitoes can be sexually competitive! Trends Parasitol. 2020, 36, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Pleydell, D.R.; Bouyer, J. Biopesticides improve efficiency of the sterile insect technique for controlling mosquito-driven dengue epidemics. Commun. Biol. 2019, 2, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Haramboure, M.; Labbé, P.; Baldet, T.; Damiens, D.; Gouagna, L.C.; Bouyer, J.; Tran, A. Modelling the control of Aedes Albopictus mosquitoes based on sterile males release techniques in a tropical environment. Ecol. Model. 2020, 424, 109002. [Google Scholar] [CrossRef]
- Oliva, C.F.; Jacquet, M.; Gilles, J.; Lemperiere, G.; Maquart, P.-O.; Quilici, S.; Schooneman, F.; Vreysen, M.J.B.; Boyer, S. The sterile insect technique for controlling populations of Aedes Albopictus (Diptera: Culicidae) on Reunion Island: Mating vigour of sterilized males. PLoS ONE 2012, 7, e49414. [Google Scholar] [CrossRef] [Green Version]
- Balestrino, F.; Puggioli, A.; Carrieri, M.; Bouyer, J.; Bellini, R. Quality control methods for Aedes Albopictus sterile male production. PLoS Negl. Trop. Dis. 2017, 11, e0005881. [Google Scholar] [CrossRef] [Green Version]
- Culbert, N.J.; Balestrino, F.; Dor, A.; Herranz, G.S.; Yamada, H.; Wallner, T.; Bouyer, J. A rapid quality control test to foster the development of genetic control in mosquitoes. Sci. Rep. 2018, 8, 16179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Culbert, N.J.; Somda, N.S.B.; Hamidou, M.; Soma, D.D.; Caravantes, S.; Wallner, T.; Wadaka, M.; Yamada, H.; Bouyer, J. A Rapid quality control test to foster the development of the sterile insect technique against Anopheles Arabiensis. Malar. J. 2020, 19, 1–10. [Google Scholar] [CrossRef]
- Culbert, N.J.; Lees, R.S.; Vreysen, M.J.; Darby, A.C.; Gilles, J.R. Optimised conditions for handling and transport of male Anopheles Arabiensis: Effects of low temperature, compaction, and ventilation on male quality. Entomol. Exp. Appl. 2017, 164, 276–283. [Google Scholar] [CrossRef] [Green Version]
- Culbert, N.J.; Gilles, J.R.; Bouyer, J. Investigating the impact of chilling temperature on male Aedes Aegypti and Aedes Albopictus survival. PLoS ONE 2019, 14, e0221822. [Google Scholar] [CrossRef]
- Culbert, N.; Kaiser, M.; Venter, N.; Vreysen, M.; Gilles, J.; Bouyer, J. A Standardised method of marking male mosquitoes for a small-scale release. Parasites Vectors 2019, 13, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouyer, J.; Culbert, N.J.; Dicko, A.H.; Pacheco, M.G.; Virginio, J.; Pedrosa, M.C.; Garziera, L.; Pinto, A.T.M.; Klaptocz, A.; Germann, J.; et al. Field performance of sterile male mosquitoes released from an uncrewed aerial vehicle. Sci. Robot. 2020, 5, eaba6251. [Google Scholar] [CrossRef]
- Bouyer, J.; Yamada, H.; Pereira, R.; Bourtzis, K.; Vreysen, M.J.B. Phased conditional approach for mosquito management using sterile insect technique. Trends Parasitol. 2020, 36, 325–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellini, R.; Medici, A.; Puggioli, A.; Balestrino, F.; Carrieri, M. Pilot field trials with Aedes Albopictus irradiated sterile males in italian urban areas. J. Med. Entomol. 2013, 50, 317–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyaloo, D.P.; Bouyer, J.; Facknath, S.; Bheecarry, A. Pilot suppression trial of Aedes Albopictus mosquitoes through an integrated vector management strategy including the sterile insect technique in Mauritius. bioRxiv 2020. biorxiv:284968/2020. [Google Scholar]
- Bouyer, J.; Lefrançois, T. Boosting the sterile insect technique to control mosquitoes. Trends Parasitol. 2014, 30, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Bouyer, J.; Chandre, F.; Gilles, J.; Baldet, T. Alternative vector control methods to manage the zika virus outbreak: More haste, less speed. Lancet Glob. Health 2016, 4, e364. [Google Scholar] [CrossRef] [Green Version]
- Hendrichs, J.; Pereira, R.; Vreysen, M.J.B. (Eds.) Area-Wide Integrated Pest Management: Development and Field Application, 1st ed.; CRC Press: Boca Raton, FL, USA, 2021; ISBN 978-1-00-316923-9. [Google Scholar]
- Vreysen, M.J.B.; Seck, M.T.; Sall, B.; Mbyaye, A.G.; Bassene, M.; Fall, A.G.; Lo, M.; Bouyer, J. Area-wide integrated pest management of a Glossina palpalis gambiensis population from the Niayes area of Senegal: A review of operational research in support of an operational phased conditional approach. In Area-Wide Integrated Pest Management: Development and Field Application; Hendrichs, J., Pereira, R., Vreysen, M.J.B., Eds.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Diall, O.; Cecchi, G.; Wanda, G.; Argilés-Herrero, R.; Vreysen, M.J.; Cattoli, G.; Viljoen, G.J.; Mattioli, R.; Bouyer, J. Developing a progressive control pathway for african animal trypanosomosis. Trends Parasitol. 2017, 33, 499–509. [Google Scholar] [CrossRef] [Green Version]
- Bello-Rivera, A.; Pereira, R.; Enkerlin, W.; Bloem, S.; Bloem, K.; Height, S.; Carpenter, J.; Zimmermann, H.; Sanchez-Anguiano, H.; Zetina-Rodriquez, R.; et al. Successful area-wide programme that eradicated outbreaks of the invasive cactus moth in Mexico. In Area-Wide Ontegrated Pest Management: Development and Field Application; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Suckling, D.M.; Charles, J.; Allan, D.; Chaggan, A.; Barrington, A.M.; Burnip, G.M.; El-sayed, A.M. Performance of irradiated Teia Anartoides (Lepidoptera: Lymantriidae) in urban Auckland, New Zealand. J. Econ. Entomol. 2005, 98, 1531–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zavala-Lopez, J.; Marte-Diaz, G.; Martinez-Pujols, F. Successful Area-Wide Eradication of the invading mediterranean fruit fly in the Dominican Republic. In Area-Wide Ontegrated Pest Management: Development and Field Application; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]









| Project Number | Coordination Research Projects | Special Issue (Reference) |
|---|---|---|
| G3.40.01 | Development of Standardized Mass Rearing Systems for Male Mosquitoes (2005–2011) | International Journal of Tropical Insect Science|Volume 34, supplement issue 1 (springer.com) |
| D4.20.12 | Improving SIT for Tsetse Flies through Research on their Symbionts and Pathogens (2007–2012) | Journal of Invertebrate Pathology|Tse Tse Fly Symposium|ScienceDirect.com by Elsevier |
| G3.40.02 | Biology of Male Mosquitoes in Relation to Genetic Control Programs (2008–2013) | Acta Tropica|Biology and behaviour of male mosquitoes in relation to new approaches to control disease transmitting mosquitoes|ScienceDirect.com by Elsevier |
| D4.20.13 | Applying Population Genetics and GIS for Managing Livestock Insect Pests (2008–2013) | Acta Tropica|Applying GIS and population genetics for managing livestock insect pests: Case studies of tsetse and screwworm flies|ScienceDirect.com by Elsevier |
| D4.10.22 | Increasing the Efficiency of Lepidoptera SIT Through Enhanced Quality Control (2009–2014) | Volume 99 Special Issue 1|Florida Entomologist (bioone.org) |
| D6.20.08 | Development of Generic Irradiation Doses for Quarantine Treatments (2009–2014, managed by Food and Environmental Protection Subprogram) | Vol. 99, Special Issue 2 (October 2016)|Florida Entomologist (flvc.org) |
| D4.20.14 | Development and Evaluation of Improved Strains of Insect Pests for SIT (2009–2014) | BMC Genomic Data|Volume 15, supplement issue 2 (springer.com) |
| D4.20.23 | Resolution of Cryptic Species Complexes of Tephritid Pests to Overcome Constraints to SIT Application and International Trade (2010–2015) | Resolution of Cryptic Species Complexes of Tephritid Pests to Enhance SIT Application and Facilitate International Trade (pensoft.net) |
| D4.10.24 | Use of Symbiotic Bacteria to Reduce Mass-Rearing Costs and Increase Mating Success in Selected Fruit Pests in Support of SIT Application (2012–2017) | Proceedings of an FAO/IAEA coordinated research project on use of symbiotic bacteria to reduce mass-rearing costs and increase mating success in selected fruit pests in support of SIT application (biomedcentral.com) |
| D4.20.15 | Enhancing Vector Refractoriness to Trypanosome Infection (2013–2018) | BMC Microbiology|Enhancing vector refractoriness to trypanosome infection (biomedcentral.com) |
| D4.40.01 | Exploring Genetic, Molecular, Mechanical and Behavioral Methods of Sex Separation in Mosquitoes (2013–2018) | Parasites & Vectors|Exploring genetic, molecular, mechanical and behavioural methods of sex separation in mosquitoes (biomedcentral.com) |
| D4.20.16 | Comparing Rearing Efficiency and Competitiveness of Sterile Male Strains Produced by Genetic, Transgenic or Symbiont-based Technologies (2015–2020) | BMC Genomic Data|Comparing rearing efficiency and competitiveness of sterile male strains produced by genetic, transgenic or symbiont-based technologies (biomedcentral.com) |
| D4.10.25 | Dormancy Management to Enable Mass-rearing and Increase Efficacy of Sterile Insects and Natural Enemies (2014–2019) | In preparation |
| D4.40.02 | Mosquito Handling, Transport, Release and Male Trapping Methods (2015–2020) | In preparation |
| D4.10.26 | Improved Field Performance of Sterile Male Lepidoptera to Ensure Success in SIT Programmes (2016–2021) | - |
| D4.30.03 | Integration of the SIT with Biocontrol for Greenhouse Insect Pest Management (2017–2022) | - |
| D4.20.17 | Improvement of Colony Management in Insect Mass-rearing for SIT Applications (2018–2023) | - |
| D4.10.27 | Assessment of Simultaneous Application of SIT and MAT to Enhance Bactrocera Fruit Fly Management (2019–2024) | - |
| D4.40.03 | Generic Approach for the Development of Genetic Sexing Strains for SIT Applications (2019–2024) | - |
| D4.40.04 | Mosquito Radiation, Sterilization and Quality Control (2020–2025) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vreysen, M.J.B.; Abd-Alla, A.M.M.; Bourtzis, K.; Bouyer, J.; Caceres, C.; de Beer, C.; Oliveira Carvalho, D.; Maiga, H.; Mamai, W.; Nikolouli, K.; et al. The Insect Pest Control Laboratory of the Joint FAO/IAEA Programme: Ten Years (2010–2020) of Research and Development, Achievements and Challenges in Support of the Sterile Insect Technique. Insects 2021, 12, 346. https://doi.org/10.3390/insects12040346
Vreysen MJB, Abd-Alla AMM, Bourtzis K, Bouyer J, Caceres C, de Beer C, Oliveira Carvalho D, Maiga H, Mamai W, Nikolouli K, et al. The Insect Pest Control Laboratory of the Joint FAO/IAEA Programme: Ten Years (2010–2020) of Research and Development, Achievements and Challenges in Support of the Sterile Insect Technique. Insects. 2021; 12(4):346. https://doi.org/10.3390/insects12040346
Chicago/Turabian StyleVreysen, Marc J. B., Adly M. M. Abd-Alla, Kostas Bourtzis, Jeremy Bouyer, Carlos Caceres, Chantel de Beer, Danilo Oliveira Carvalho, Hamidou Maiga, Wadaka Mamai, Katerina Nikolouli, and et al. 2021. "The Insect Pest Control Laboratory of the Joint FAO/IAEA Programme: Ten Years (2010–2020) of Research and Development, Achievements and Challenges in Support of the Sterile Insect Technique" Insects 12, no. 4: 346. https://doi.org/10.3390/insects12040346
APA StyleVreysen, M. J. B., Abd-Alla, A. M. M., Bourtzis, K., Bouyer, J., Caceres, C., de Beer, C., Oliveira Carvalho, D., Maiga, H., Mamai, W., Nikolouli, K., Yamada, H., & Pereira, R. (2021). The Insect Pest Control Laboratory of the Joint FAO/IAEA Programme: Ten Years (2010–2020) of Research and Development, Achievements and Challenges in Support of the Sterile Insect Technique. Insects, 12(4), 346. https://doi.org/10.3390/insects12040346