Just a Fragment of Undescribed Diversity: Twenty New Oriental and Palearctic Species of Sciaroidea (Diptera), including DNA Sequence Data and Two New Fossil Genera
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Family Bolitophilidae
3.2. Family Cecidomyiidae
3.2.1. Genus Catocha Haliday, 1833
3.2.2. Genus Planetella Westwood, 1840
3.3. Family Diadocidiidae
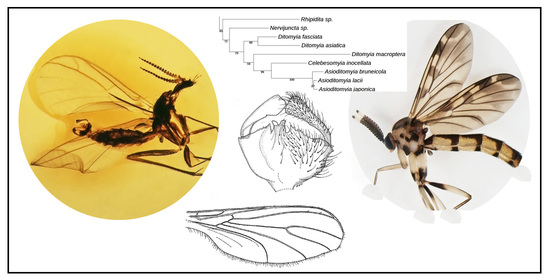
3.4. Family Ditomyiidae
3.4.1. Genus Asioditomyia Saigusa, 1973
3.4.2. Burmasymmerus gen. nov.
3.4.3. Genus Celebesomyia Saigusa, 1973
3.4.4. Genus Ditomyia Winnertz, 1846
3.5. Family Keroplatidae
3.5.1. Genus Chetoneura Colless, 1962
3.5.2. Genus Euceroplatus Edwards, 1929
3.5.3. Genus Platyceridion Tollet, 1955
3.5.4. Genus Setostylus Matile, 1990
3.5.5. Genus Terocelion Ševčík, 2012
3.6. Family Mycetophilidae
3.6.1. Genus Hadroneura Lundström, 1906
3.6.2. Genus Paratinia Mik, 1874
3.7. Sciaroidea Incertae Sedis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hebert, P.D.N.; Ratnasingham, S.; Zakharov, E.V.; Telfer, A.C.; Levesque-Beaudin, V.; Milton, M.A.; Pedersen, S.; Jannetta, P.; DeWaard, J.R. Counting animal species with DNA barcodes: Canadian insects. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150333. [Google Scholar] [CrossRef] [Green Version]
- Ševčík, J.; Kaspřák, D.; Mantič, M.; Fitzgerald, S.; Ševčíková, T.; Tóthová, A.; Jaschhof, M. Molecular phylogeny of the megadiverse insect infraorder Bibionomorpha sensu lato (Diptera). PeerJ 2016, 4, e2563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantič, M.; Sikora, T.; Burdíková, N.; Blagoderov, V.; Kjærandsen, J.; Kurina, O.; Ševčík, J. Hidden in plain sight: Comprehensive molecular phylogeny of Keroplatidae and Lygistorrhinidae (Diptera) reveals parallel evolution and leads to a revised family classification. Insects 2020, 11, 348. [Google Scholar] [CrossRef] [PubMed]
- Ševčík, J.; Bechev, D.; Hippa, H. New Oriental species of Gnoristinae (Diptera: Mycetophilidae) with pectinate antennae. Acta Entomol. Mus. Natl. Pragae 2011, 51, 687–696. [Google Scholar]
- Ševčík, J.; Hippa, H.; Wahab, R.A. Diversity of Manota Williston (Diptera, Mycetophilidae) in Ulu Temburong National Park, Brunei. ZooKeys 2014, 428, 57–77. [Google Scholar] [CrossRef] [Green Version]
- Blagoderov, V.; Hippa, H.; Ševčík, J. Asiorrhina, a new Oriental genus of Lygistorrhinidae (Diptera: Sciaroidea) and its phylogenetic position. Zootaxa 2009, 2295, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Ševčík, J.; Mantič, M.; Blagoderov, V. Two new genera of Keroplatidae (Diptera), with an updated key to the World genera of Keroplatini. Acta Entomol. Mus. Natl. Pragae 2015, 55, 387–399. [Google Scholar]
- Vilkamaa, P.; Rudzinski, H.-G.; Burdíková, N.; Ševčík, J. Phylogenetic position of Aerumnosa Mohrig (Diptera, Sciaridae) as revealed by multigene analysis, with the description of four new Oriental species. Zootaxa 2018, 4399, 248–260. [Google Scholar] [CrossRef]
- Vilkamaa, P.; Halenius, P.; Ševčík, J. Review of Pseudoaerumnosa Rudzinski (Diptera, Sciaridae), with the description of twenty-four new species. Zootaxa 2019, 4656, 1–42. [Google Scholar] [CrossRef]
- Jaschhof, M. A review of world Diallactiini (Diptera, Cecidomyiidae, Winnertziinae), with the description of six new genera and seventeen new species. Zootaxa 2016, 4127, 201–244. [Google Scholar] [CrossRef]
- Ševčík, J.; Krzemiński, W.; Skibińska, K. Intriguing and Beautiful: Adamacrocera adami gen. et sp. nov. from the Upper Cretaceous Amber of Myanmar Represents a New Subfamily of Keroplatidae (Diptera: Bibionomorpha). Insects 2020, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Ševčík, J.; Skartveit, J.; Krzemiński, W.; Skibińska, K. A Peculiar New Genus of Bibionomorpha (Diptera) with Brachycera-Like Modification of Antennae from Mid-Cretaceous Amber of Myanmar. Insects 2021, 12, 364. [Google Scholar] [CrossRef] [PubMed]
- Blagoderov, V.; Ševčík, J. Keroplatidae (Predaceous Fungus Gnats). In Manual of Afrotropical Diptera. Nematocerous Diptera and Lower Brachycera, 2nd ed.; Kirk–Spriggs, A.H., Sinclair, B.J., Eds.; South African National Biodiversity Institute: Pretoria, South Africa, 2017; Volume 2, pp. 527–531. [Google Scholar]
- Ševčík, J.; Burdíková, N.; Kaspřák, D.; Kurina, O. Five new Palaearctic species of Docosia (Diptera: Mycetophilidae), with updated molecular phylogeny of the genus. Eur. J. Taxon. 2020, 717, 3–26. [Google Scholar] [CrossRef]
- Bechev, D.; Chandler, P.J. Catalogue of the Bolitophilidae and Diadocidiidae of the World (Insecta: Diptera). Zootaxa 2011, 2741, 38–58. [Google Scholar] [CrossRef]
- Ševčík, J.; Papp, L. Bolitophilidae (Diptera) from Taiwan: A family new to the Oriental Region. Acta Zool. Acad. Sci. Hung. 2004, 50, 55–62. [Google Scholar]
- Gagné, R.J.; Jaschhof, M. A Catalog of the Cecidomyiidae (Diptera) of the World, 5th ed.; USDA (United States Department of Agriculture): Washington, DC, USA, 2021; p. 814. Available online: https://www.ars.usda.gov/ARSUserFiles/80420580/Gagne_Jaschhof_2021_World_Cat_5th_Ed.pdf (accessed on 21 November 2021).
- Sikora, T.; Jaschhof, M.; Mantič, M.; Kaspřák, D.; Ševčík, J. Considerable congruence, enlightening conflict: Molecular analysis largely supports morphology-based hypotheses on Cecidomyiidae (Diptera) phylogeny. Zool. J. Linn. Soc. 2019, 185, 98–110. [Google Scholar] [CrossRef]
- Jaschhof, M.; Jaschhof, C. The wood midges (Diptera: Cecidomyiidae) of Fennoscandia and Denmark. Studia Dipterol. Suppl. 2009, 18, 1–333. [Google Scholar]
- Ševčík, J.; Kaspřák, D.; Mantič, M.; Ševčíková, T.; Tóthová, A. Molecular phylogeny of the fungus gnat family Diadocidiidae and its position within the infraorder Bibionomorpha (Diptera). Zool. Scr. 2014, 43, 370–378. [Google Scholar] [CrossRef]
- Papp, L.; Ševčík, J. New taxa of Diadocidiidae (Diptera) from the Oriental region. Acta Zool. Acad. Sci. Hung. 2005, 51, 329–341. [Google Scholar]
- Saigusa, T. A systematic study of the Mycetophilidae of Japan (Diptera). Part 1. A revision of the subfamily Ditomyiinae. Sieboldia 1973, 4, 167–200. [Google Scholar]
- Saigusa, T. A new genus and species of the Ditomyiinae from Celebes (Diptera: Mycetophilidae). Sieboldia 1973, 4, 217–223. [Google Scholar]
- Ansorge, J. Insekten aus dem oberen Lias von Grimmen (Vorpommern, Norddeutschland). Neue Paläont. Abhandl. 1996, 2, 1–132. [Google Scholar]
- Fitzgerald, S. Revision of the Nearctic species of Ditomyia Winnertz and a new species from the Neotropical Region (Diptera: Ditomyiidae). Zootaxa 2021, 4859, 27–42. [Google Scholar]
- Papp, L.; Ševčík, J. Sciarokeroplatinae, a new subfamily of Keroplatidae (Diptera). Acta Zool. Acad. Sci. Hung. 2005, 51, 113–123. [Google Scholar]
- Ševčík, J.; Papp, L. Microkeroplatus, a new genus of Keroplatidae (Diptera) from the Oriental region. Acta Zool. Acad. Sci. Hung. 2009, 55, 339–347. [Google Scholar]
- Ševčík, J. Terocelion gen. nov., a new Oriental genus of Keroplatidae (Diptera) with pectinate antennae. Acta Entomol. Mus. Natl. Pragae 2012, 52, 495–503. [Google Scholar]
- Ševčík, J. Pseudochetoneura gen. nov., a peculiar new genus from Ecuador, with notes on Chetoneura (Diptera: Keroplatidae). Acta Entomol. Mus. Nat. Prag. 2012, 52, 281–288. [Google Scholar]
- Chandler, P.; Matile, L. A new species of Platyceridion Tollet (Diptera, Keroplatidae) with a larva predatory in ant infested internodes of Humboldtia laurifolia Vahl. Studia Dipterol. 1998, 5, 163–173. [Google Scholar]
- Xu, H.; Cao, J.; Zhou, Z.; Wu, H.; Huang, Z. First record of the tribe Keroplatini from China, with descriptions of two new species (Diptera: Keroplatidae). Zootaxa 2007, 1497, 35–40. [Google Scholar] [CrossRef]
- Kaspřák, D.; Kerr, P.; Sýkora, V.; Tóthová, A.; Ševčík, J. Molecular phylogeny of the fungus gnat subfamilies Gnoristinae and Mycomyinae, and their position within Mycetophilidae (Diptera). Syst. Ent. 2019, 44, 128–138. [Google Scholar] [CrossRef]
- Hippa, H.; Ševčík, J. Notes on Oriental and Australasian Manotinae (Diptera, Mycetophilidae), with the description of thirteen new species. Zootaxa 2010, 2333, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Papp, L.; Ševčík, J. Eight new Oriental and Australasian species of Leptomorphus (Diptera: Mycetophilidae). Acta Zool. Acad. Sci. Hung. 2011, 57, 139–159. [Google Scholar]
- Kerr, P. Six new species of Acomoptera from North America (Diptera, Mycetophilidae). ZooKeys 2011, 137, 41–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Søli, G.E.E. The adult morphology of Mycetophilidae (s.str.), with a tentative phylogeny of the family (Diptera, Sciaroidea). Ent. Scand. Suppl. 1997, 50, 5–55. [Google Scholar]
- Vockeroth, J.R. New genera and species of Mycetophilidae (Diptera) from the Holarctic region, with notes on other species. Can. Entomol. 1980, 112, 529–544. [Google Scholar] [CrossRef]
- Ševčík, J. 2004 New data on Sciaroidea (Diptera) from the Czech and Slovak Republics, with descriptions of seven new species of Mycetophilidae. Čas. Slez. Muz. Opava. (A) 2004, 53, 49–74. [Google Scholar]
- Taber, S. The Previously Unknown Female of the Fungus Gnat genus Paratinia Mik (Diptera: Mycetophilidae) with Notes on Nearctic Males. Southwest. Entomol. 2015, 40, 131–140. [Google Scholar] [CrossRef]
- Chandler, P. Heterotricha Loew and allied genera (Diptera: Sciaroidea): Offshoots of the stem group of Mycetophilidae and/or Sciaridae? Ann. Soc. Entomol. Fr. (Nouvelle Série) 2002, 38, 101–144. [Google Scholar]
- Hippa, H.; Ševčík, J. Notes on Nepaletricha (Diptera: Sciaroidea incertae sedis), with description of three new species from India and Vietnam. Acta Entomol. Mus. Nat. Pragae 2014, 54, 729–739. [Google Scholar]
- Jaschhof, M. Phylogeny and classification of the Sciaroidea (Diptera: Bibionomorpha): Where do we stand after Amorim & Rindal (2007)? Beitr. Entomol. 2011, 61, 455–463. [Google Scholar]
- Hippa, H.; Chandler, P.J.; Papp, L. Review of The Genus Nepaletricha Chandler (Diptera, Rangomaramidae), with description of new species from Thailand and Vietnam. Zootaxa 2009, 2174, 18–26. [Google Scholar] [CrossRef]

















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ševčík, J.; Hippa, H.; Burdíková, N. Just a Fragment of Undescribed Diversity: Twenty New Oriental and Palearctic Species of Sciaroidea (Diptera), including DNA Sequence Data and Two New Fossil Genera. Insects 2022, 13, 19. https://doi.org/10.3390/insects13010019
Ševčík J, Hippa H, Burdíková N. Just a Fragment of Undescribed Diversity: Twenty New Oriental and Palearctic Species of Sciaroidea (Diptera), including DNA Sequence Data and Two New Fossil Genera. Insects. 2022; 13(1):19. https://doi.org/10.3390/insects13010019
Chicago/Turabian StyleŠevčík, Jan, Heikki Hippa, and Nikola Burdíková. 2022. "Just a Fragment of Undescribed Diversity: Twenty New Oriental and Palearctic Species of Sciaroidea (Diptera), including DNA Sequence Data and Two New Fossil Genera" Insects 13, no. 1: 19. https://doi.org/10.3390/insects13010019
APA StyleŠevčík, J., Hippa, H., & Burdíková, N. (2022). Just a Fragment of Undescribed Diversity: Twenty New Oriental and Palearctic Species of Sciaroidea (Diptera), including DNA Sequence Data and Two New Fossil Genera. Insects, 13(1), 19. https://doi.org/10.3390/insects13010019