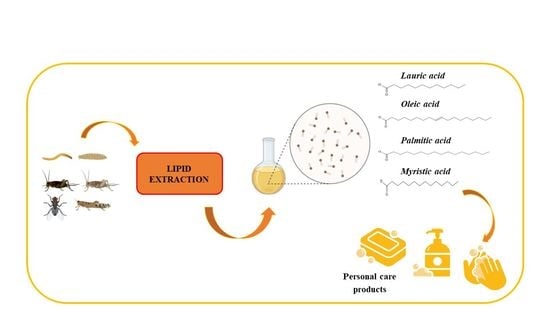
Lipids from Insects in Cosmetics and for Personal Care Products
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Insects
1.2. Lipids from Insects
2. Extraction of Insect Lipids
3. Lipids in Cosmetics and Personal Care Products
3.1. Lauric Acid (C12:0)
3.2. Myristic Acid (C14:0)
3.3. Palmitic Acid (C16:0)
3.4. Oleic Acid (C18:1 n-9)
4. Insects as Lipids Source for Cosmetic Applications
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chapman, A.D. Numbers of Living Species in Australia and the World, 1st ed.; Australian Biological Resources Study: Camberra, Australia, 2009; pp. 1–78. [Google Scholar]
- Engel, M.S. Insect evolution. Curr. Biol. 2015, 25, R868–R872. [Google Scholar] [CrossRef] [Green Version]
- Yi, Z.; Jinchao, F.; Dayuan, X.; Weiguo, S.; Axmacher, J. Insect diversity: Addressing an important but strongly neglected research topic in China. J. Res. Ecol. 2011, 2, 380–384. [Google Scholar] [CrossRef]
- Chown, S.L.; Terblanche, J.S. Physiological Diversity in Insects: Ecological and Evolutionary Contexts. Adv. Insect Physiol. 2006, 33, 50–152. [Google Scholar]
- Sheikh, A.A.; Rehman, N.Z.; Kumar, R. Diverse adaptations in insects: A review. J. Entomol. Zool. 2007, 5, 343–350. [Google Scholar]
- Noriega, J.A.; Hortal, J.; Azcárate, F.M.; Berg, M.P.; Bonada, N.; Briones, M.J.I.; Del Toro, I.; Goulson, D.; Ibanez, S.; Landis, D.A.; et al. Research trends in ecosystem services provided by insects. Basic Appl. Ecol. 2018, 26, 8–23. [Google Scholar] [CrossRef] [Green Version]
- Mauricio da Rocha, J.R.; De Almeida, J.R.; Lins, G.A.; Durval, A. Insects As Indicators of Environmental Changing and Pollution: A Review of Appropriate Species and Their Monitoring. Holos Environ. 2010, 10, 250. [Google Scholar] [CrossRef]
- Jouquet, P.; Traoré, S.; Choosai, C.; Hartmann, C.; Bignell, D. Influence of termites on ecosystem functioning. Ecosystem services provided by termites. Eur. J. Soil Biol. 2011, 47, 215–222. [Google Scholar] [CrossRef]
- Kyerematen, R.; Acquah-Lamptey, D.; Owusu, E.H.; Anderson, R.S.; Ntiamoa-Baidu, Y. Insect Diversity of the Muni-Pomadze Ramsar Site: An Important Site for Biodiversity Conservation in Ghana. J. Insects 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Salvia, R.; Falabella, P. Bioconverter insects: A good example of circular economy, the study case of Hermetia illucens. In An Introduction to the Circular Economy, 1st ed.; Springer Nature: Basingstoke, UK, 2021; pp. 261–280. [Google Scholar]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef] [PubMed]
- Scala, A.; Cammack, J.A.; Salvia, R.; Scieuzo, C.; Franco, A.; Bufo, S.A.; Tomberlin, J.K.; Falabella, P. Rearing substrate impacts growth and macronutrient composition of Hermetia illucens (L.) (Diptera: Stratiomyidae) larvae produced at an industrial scale. Sci. Rep. 2020, 10, 19448. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2017/893 of 24 May 2017 Amending Annexes I and IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council and Annexes X, XIV and XV to Commission Regulation (EU) No 142/2011 as Regards the Provisions on Processed Animal Protein. Available online: https://eur-lex.europa.eu/legal-content/IT/TXT/?uri=uriserv%3AOJ.L_.2017.138.01.0092.01.ITA&toc=OJ%3AL%3A2017%3A138%3ATOC (accessed on 15 September 2021).
- Commission Regulation (EU) 2021/1372 of 17 August 2021 Amending Annex IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council as Regards the Prohibition to Feed Non-Ruminant Farmed Animals, Other than Fur Animals, with Protein Derived from Animals. Available online: https://eur-lex.europa.eu/legal-content/IT/TXT/?uri=CELEX:32021R1372 (accessed on 18 November 2021).
- EFSA Panel on Nutrition; Novel Foods and Food Allergens (NDA); Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; et al. Safety of dried yellow mealworm (Tenebrio molitor larva) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06343. [Google Scholar] [CrossRef] [PubMed]
- Mertenat, A.; Diener, S.; Zurbrügg, C. Black Soldier Fly biowaste treatment–Assessment of global warming potential. Waste Manag. 2019, 84, 173–181. [Google Scholar] [CrossRef]
- Halloran, A.; Hanboonsong, Y.; Roos, N.; Bruun, S. Life cycle assessment of cricket farming in north-eastern Thailand. J. Clean. Prod. 2017, 156, 83–94. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; van Broekhoven, S.; van Huis, A.; van Loon, J.J.A. Feed Conversion, Survival and Development, and Composition of Four Insect Species on Diets Composed of Food By-Products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niyonsaba, H.H.; Höhler, J.; Kooistra, J.; Van der Fels-Klerx, H.J.; Meuwissen, M.P.M. Profitability of insect farms. J. Insects Food Feed 2021, 7, 923–934. [Google Scholar] [CrossRef]
- Research and Markets, 2019. Insect Feed Market–Growth, Trends and Forecasts (2020–2025). Available online: https://www.researchandmarkets.com/reports/4904389/insect-feed-marketgrowth-trends-and-forecasts (accessed on 3 December 2021).
- International Platform of Insects for Food and Feed (IPIFF). Ensuring High Standards of Animal Welfare in Insect Production; IPIFF: Brussels, Belgium, 2019; Available online: https://tinyurl.com/yd88udze (accessed on 3 December 2021).
- Macombe, C.; Le Feon, S.; Aubin, J.; Maillard, F. Marketing and social effects of industrial scale insect value chains in Europe: Case of mealworm for feed in France. J. Insects Food Feed 2019, 5, 215–224. [Google Scholar] [CrossRef]
- Mancuso, T.; Pippinato, L.; Gasco, L. The European insects sector and its role in the provision of green proteins in feed supply. Calitatea 2019, 20, 374–381. [Google Scholar]
- Tomberlin, J.K.; Sheppard, D.C.; Joyce, J.A. Selected life-history traits of black soldier flies (Diptera: Stratiomyidae) reared on three artificial diets. Annal. Entomol. Soc. Am. 2002, 95, 379–386. [Google Scholar] [CrossRef]
- Downer, R.G.H.; Matthews, J.R. Patterns of Lipid Distribution and Utilisation in Insects. Am. Zool. 1976, 16, 733–745. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect Fat Body: Energy, Metabolism, and Regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Yu, X.; Feng, Q. Fat Body Biology in the Last Decade. Annu. Rev. Entomol. 2019, 64, 315–333. [Google Scholar] [CrossRef] [PubMed]
- Gäde, G. Regulation of intermediary metabolism and water balance of insects by neuropeptides. Ann. Rev. Entomol. 2004, 49, 93–113. [Google Scholar] [CrossRef]
- Zheng, H.; Yang, X.; Xi, Y. Fat body remodeling and homeostasis control in Drosophila. Life Sci. 2016, 167, 22–31. [Google Scholar] [CrossRef] [PubMed]
- da Silva Lucas, J.A.; de Oliveira, M.L.; da Rocha, M.; Prentice, C. Edible insects: An alternative of nutritional, functional and bioactive compounds. Food Chem. 2020, 311, 126022. [Google Scholar] [CrossRef]
- Xiaoming, C.; Ying, F.; Hong, Z.; Zhiyong, C. Review of the nutritive value of edible insects. In Forest Insects as Food: Humans Bite Back, Proceedings of the a Workshop on Asia-Pacific Resources and Their Potential for Development, 19–21 February 2008, Chiang Mai, Thailand; Food and Agriculture Organization of the United Nations (FAO): Bangkok, Thailand, 2010; pp. 85–92. [Google Scholar]
- Lease, H.M.; Wolf, B.O. Lipid content of terrestrial arthropods in relation to body size, phylogeny, ontogeny and sex. Physiol. Entomol. 2011, 36, 29–38. [Google Scholar] [CrossRef]
- Bentz, B.J. Mountain pine beetle population sampling: Inferences from Lindgren pheromone traps and tree emergence cages. Can. J. For. Res. 2006, 36, 351–360. [Google Scholar] [CrossRef]
- Gilbert, L.I.; Schneiderman, H.A. The content of juvenile hormone and lipid in lepidoptera: Sexual differences and developmental changes. Gen. Comp. Endocrinol. 1961, 1, 453–472. [Google Scholar] [CrossRef]
- Jackson, L.L.; Arnold, M.T.; Blomquist, G.J. Surface lipids of Drosophila melanogaster: Comparison of the lipids from female and male wild type and sex-linked yellow mutant. Insect Biochem. 1981, 11, 87–91. [Google Scholar] [CrossRef]
- Reddiex, A.J.; Gosden, T.P.; Bonduriansky, R.; Chenoweth, S.F. Sex-specific fitness consequences of nutrient intake and the evolvability of diet preferences. Am. Nat. 2013, 182, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Beenakkers, A.M.; Van der Horst, D.J.; Van Marrewijk, W.J.A. Insect lipids and lipoproteins, and their role in physiological processes. Prog. Lipid Res. 1985, 24, 19–67. [Google Scholar] [CrossRef]
- Alnajim, I.; Du, X.; Lee, B.; Agarwal, M.; Liu, T.; Ren, Y. New Method of Analysis of Lipids in Tribolium castaneum (Herbst) and Rhyzopertha dominica (Fabricius) Insects by Direct Immersion Solid-Phase Microextraction (DI-SPME) Coupled with GC–MS. Insects 2019, 10, 363. [Google Scholar] [CrossRef] [Green Version]
- Berson, J.D.; Simmons, L.W. Female cuticular hydrocarbons can signal indirect fecundity benefits in an insect. Evolution 2019, 73, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Ferveur, J.F.; Cortot, J.; Rihani, K.; Cobb, M.; Everaerts, C. Desiccation resistance: Effect of cuticular hydrocarbons and water content in Drosophila melanogaster adults. PeerJ 2018, 6, e4318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwangbo, D.S.; Gersham, B.; Tu, M.P.; Palmer, M.; Tatar, M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature 2004, 429, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Ketterman, A.J.; Saisawang, C.; Wongsantichon, J. Insect glutathione transferases. Drug Metab. Rev. 2011, 43, 253–265. [Google Scholar] [CrossRef]
- Hahn, D.A.; Denlinger, D.L. Meeting the energetic demands of insect diapause: Nutrient storage and utilization. J. Insect Physiol. 2007, 53, 760–773. [Google Scholar] [CrossRef]
- Hahn, D.A.; Denlinger, D.L. Energetics of Insect Diapause. Annu. Rev. Entomol. 2011, 56, 103–121. [Google Scholar] [CrossRef]
- Sinclair, B.J.; Marshall, K.E. The many roles of fats in overwintering insects. J. Exp. Biol. 2018, 7, 221. [Google Scholar] [CrossRef] [Green Version]
- Franco, A.; Scieuzo, C.; Salvia, R.; Petrone, A.M.; Tafi, E.; Moretta, A.; Schmitt, E.; Falabella, P. Lipids from Hermetia illucens, an Innovative and Sustainable Source. Sustainability 2021, 13, 10198. [Google Scholar] [CrossRef]
- Sánchez, M.; Gómez, C.; Avendaño, C.; Harmsen, I.; Ortiz, D.; Ceballos, R.; Villamizar-Sarmiento, M.G.; Oyarzun-Ampuero, F.; Wacyk, J.; Valenzuela, C. House fly (Musca domestica) larvae meal as an ingredient with high nutritional value: Microencapsulation and improvement of organoleptic characteristics. Food Res. Int. 2021, 145, 110423. [Google Scholar] [CrossRef]
- Paul, A.; Frederich, M.; Megido, R.C.; Alabi, T.; Malik, P.; Uyttenbroeck, R.; Francis, F.; Blecker, C.; Haubruge, E.; Lognay, G.; et al. Insect fatty acids: A comparison of lipids from three Orthopterans and Tenebrio molitor L. larvae. J. Asia Pac. Entomol. 2017, 20, 337–340. [Google Scholar] [CrossRef]
- Roncolini, A.; Milanović, V.; Aquilanti, L.; Cardinali, F.; Garofalo, C.; Sabbatini, R.; Clementi, F.; Belleggia, L.; Pasquini, M.; Mozzon, M.; et al. Lesser mealworm (Alphitobius diaperinus) powder as a novel baking ingredient for manufacturing high-protein, mineral-dense snacks. Food Res. Int. 2020, 131, 109031. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, E.; Baraniak, B.; Karaś, M.; Rybczyńska, K.; Jakubczyk, A. Selected species of edible insects as a source of nutrient composition. Food Res. Int. 2015, 77, 460–466. [Google Scholar] [CrossRef]
- Soares Araújo, R.R.; dos Santos Benfica, T.A.R.; Ferraz, V.P.; Moreira Santos, E. Nutritional composition of insects Gryllus assimilis and Zophobas morio: Potential foods harvested in Brazil. J. Food Compos. Anal. 2019, 76, 22–26. [Google Scholar] [CrossRef]
- Lal, J.J.; Sreeranjit Kumar, C.V.; Indira, M. Coconut Palm. In Encyclopedia of Food Sciences and Nutrition; Elsevier: Amsterdam, The Netherlands, 2003; pp. 1464–1475. [Google Scholar]
- Aluyor, E.O.; Oboh, I.O. Preservatives: Traditional Preservatives–Vegetable Oils. In Encyclopedia of Food Microbiology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 137–140. [Google Scholar]
- Mancini, A.; Imperlini, E.; Nigro, E.; Montagnese, C.; Daniele, A.; Orrù, S.; Buono, P. Biological and Nutritional Properties of Palm Oil and Palmitic Acid: Effects on Health. Molecules 2015, 20, 17339–17361. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, A.J. Lauric Oils. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 517–522. [Google Scholar]
- Marcus, J.B. Lipids Basics: Fats and Oils in Foods and Health. In Culinary Nutrition; Elsevier: Amsterdam, The Netherlands, 2013; pp. 231–277. [Google Scholar]
- Tzompa-Sosa, D.A.; Yi, L.; van Valenberg, H.J.F.; van Boekel, M.A.J.S.; Lakemond, C.M.M. Insect lipid profile: Aqueous versus organic solvent-based extraction methods. Food Res. Int. 2014, 62, 1087–1094. [Google Scholar] [CrossRef]
- Laroche, M.; Perreault, V.; Marciniak, A.; Gravel, A.; Chamberland, J.; Doyen, A. Comparison of Conventional and Sustainable Lipid Extraction Methods for the Production of Oil and Protein Isolate from Edible Insect Meal. Foods 2019, 8, 572. [Google Scholar] [CrossRef] [Green Version]
- Smets, R.; Verbinnen, B.; Van De Voorde, I.; Aerts, G.; Claes, J.; Van Der Borght, M. Sequential Extraction and Characterisation of Lipids, Proteins, and Chitin from Black Soldier Fly (Hermetia illucens) Larvae, Prepupae, and Pupae. Waste Biomass Valorization 2020, 11, 6455–6466. [Google Scholar] [CrossRef]
- Hadj Saadoun, J.; Montevecchi, G.; Zanasi, L.; Bortolini, S.; Macavei, L.I.; Masino, F.; Maistrello, L.; Antonelli, A. Lipid profile and growth of black soldier flies (Hermetia illucens, Stratiomyidae) reared on by-products from different food chains. J. Sci. Food Agric. 2020, 100, 3648–3657. [Google Scholar] [CrossRef]
- Obeng, A.K.; Atuna, R.A.; Aihoon, S. Proximate composition of housefly (Musca domestica) maggots cultured on different substrates as potential feed for Tilapia (Oreochromis niloticus). Int. J. Multidiscip. Res. Dev. 2015, 2, 102–103. [Google Scholar]
- Yi, L.; Lakemond, C.M.M.; Sagis, L.M.C.; Eisner-Schadler, V.; van Huis, A.; van Boekel, M.A.J.S. Extraction and characterisation of protein fractions from five insect species. Food Chem. 2013, 141, 3341–3348. [Google Scholar] [CrossRef]
- Rudyk, S.; Spirov, P.; Hussain, S. Effect of co-solvents on SC-CO2 extraction of crude oil by consistency test. J. Supercrit. Fluids 2014, 91, 15–23. [Google Scholar] [CrossRef]
- Purschke, B.; Scheibelberger, R.; Axmann, S.; Adler, A.; Jäger, H. Impact of substrate contamination with mycotoxins, heavy metals and pesticides on the growth performance and composition of black soldier fly larvae (Hermetia illucens) for use in the feed and food value chain. Food Addit. Contam. Part A 2017, 34, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Wolf, D.; Gutzeit, H.O. The black soldier fly, Hermetia illucens—A promising source for sustainable production of proteins, lipids and bioactive substances. Z. Nat. C 2017, 72, 351–363. [Google Scholar] [CrossRef]
- Mai, H.C.; Dao, N.D.; Lam, T.D.; Nguyen, B.V.; Nguyen, D.C.; Bach, L.G. Purification Process, Physicochemical Properties, and Fatty Acid Composition of Black Soldier Fly (Hermetia illucens Linnaeus) Larvae Oil. J. Am. Oil Chem. Soc. 2019, 96, 1303–1311. [Google Scholar] [CrossRef]
- Pomeranz, Y.; Meloan, C.E. Food Analysis; Springer: Boston, MA, USA, 1995; ISBN 978-1-4615-7000-4. [Google Scholar]
- Wongkittipong, R.; Prat, L.; Damronglerd, S.; Gourdon, C. Solid–liquid extraction of andrographolide from plants—Experimental study, kinetic reaction and model. Sep. Purif. Technol. 2004, 40, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Available online: www.eawag.ch (accessed on 10 November 2021).
- Salomone, R.; Saija, G.; Mondello, G.; Giannetto, A.; Fasulo, S.; Savastano, D. Environmental impact of food waste bioconversion by insects: Application of Life Cycle Assessment to process using Hermetia illucens. J. Clean. Prod. 2017, 140, 890–905. [Google Scholar] [CrossRef]
- Sosa, D.A.T.; Fogliano, V. Potential of Insect-Derived Ingredients for Food Applications. In Insect Physiology and Ecology; InTech Open: London, UK, 2017; pp. 215–232. [Google Scholar]
- Khosrowpour, Z.; Ahmad Nasrollahi, S.; Ayatollahi, A.; Samadi, A.; Firooz, A. Effects of four soaps on skin trans-epidermal water loss and erythema index. J. Cosmet. Dermatol. 2019, 18, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Burr, G.O.; Burr, M.M. On the nature and role of the fatty acids essential in nutrition. J. Biol. Chem. 1930, 86, 587–621. [Google Scholar] [CrossRef]
- Hansen, A.E.; Haggard, M.E.; Boelsche, A.N.; Adam, D.J.D.; Wiese, H.F. Essential Fatty Acids in Infant Nutrition. J. Nutr. 1958, 66, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Prottey, C.; Hartop, P.J.; Press, M. Correction of the Cutaneous Manifestations of Essential Fatty Acid Deficiency in Man by Application of Sunflower-Seed Oil to the Skin. J. Investig. Dermatol. 1975, 64, 228–234. [Google Scholar] [CrossRef] [Green Version]
- Burr, G.O.; Burr, M.M. A New Deficiency Disease Produced by the Rigid Exclusion of Fat from the Diet. Nutr. Rev. 2009, 31, 148–149. [Google Scholar] [CrossRef]
- Prakash, L.; Majeed, M. Natural ingredients for anti-ageing skin care. Househ. Pers. Care Today 2009, 2, 44–46. [Google Scholar]
- Plannthin, D.K. Animal Ethics and Welfare in the Fashion and Lifestyle Industries. In Green Fashion; Springer Science + Business Media: Berlin, Germany, 2016; pp. 49–122. [Google Scholar]
- Verheyen, G.R.; Ooms, T.; Vogels, L.; Vreysen, S.; Bovy, A.; Van Miert, S.; Meersman, F. Insects as an Alternative Source for the Production of Fats for Cosmetics. J. Cosmet. Sci. 2018, 69, 187–202. [Google Scholar] [PubMed]
- Windholz, M.; Budavari, S.; Blumetti, R.F.; Otterbein, E.S. The Merck Index; Merck & Co. Inc.: Rahway, NJ, USA, 1983; p. 1051. [Google Scholar]
- Swern, D. Bailey’s Industrial Oil and Fat Products, 4th ed.; John Wiley & Sons: New York, NY, USA, 1979; p. 1. [Google Scholar]
- Food Chemicals Codex (FCC), 3rd ed.; National Academy Press: Washington, DC, USA, 1981.
- Fassett, D.W.; Irish, D.D. (Eds.) Industrial Hygiene and Toxicology, 2nd ed.; Toxicology; Interscience Publishers: New York, NY, USA, 1963; Volume 2. [Google Scholar]
- SpecialChem. Available online: https://cosmetics.specialchem.com (accessed on 1 October 2021).
- Becker, L.C.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; Andersen, F.A. Final Report of the Amended Safety Assessment of Myristic Acid and Its Salts and Esters as Used in Cosmetics. Int. J. Toxicol. 2010, 29, 162S–186S. [Google Scholar] [CrossRef]
- Gosselin, R.E.; Hodge, H.C.; Smith, R.P.; Gleason, M.N. Clinical Toxicology of Commercial Products; Acute Poisoning 1; Williams and Wilkins Co.: Baltimore, MD, USA, 1976. [Google Scholar]
- Hawley, G.G. Condensed Chemical Dictionary, 9th ed.; Van Nostrand Reinhold Co.: New York, NY, USA, 1977. [Google Scholar]
- Food and Drug Administration (FDA). Cosmetic Product Formulation Data; FDA Computer Printout: Silver Spring, MD, USA, 1981.
- Osol, A. (Ed.) Remington’s Pharmaceutical Sciences, 16th ed.; Mack Publ. Co.: Easton, PA, USA, 1980. [Google Scholar]
- Barragán-Fonseca, K.; Pineda-Mejia, J.; Dicke, M.; van Loon, J.J.A. Performance of the Black Soldier Fly (Diptera: Stratiomyidae) on Vegetable Residue-Based Diets Formulated Based on Protein and Carbohydrate Contents. J. Econ. Entomol. 2018. [Google Scholar] [CrossRef]
- Gold, M.; Binggeli, M.; Kurt, F.; de Wouters, T.; Reichlin, M.; Zurbrügg, C.; Mathys, A.; Kreuzer, M. Novel Experimental Methods for the Investigation of Hermetia illucens (Diptera: Stratiomyidae) Larvae. J. Insect Sci. 2020, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, M.; Zheng, L.; Liu, Y.; Zhang, Y.; Yu, Z.; Ma, Z.; Li, Q. Simultaneous utilization of glucose and xylose for lipid accumulation in black soldier fly. Biotechnol. Biofuels 2015, 8, 117. [Google Scholar] [CrossRef] [Green Version]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Wu, J.W.; Chen, M.; Peng, W.F. Study on the nutritional value of the housefly larva fed with pig manure. J. Guiyang Med. Coll. 2001, 26, 377–379. [Google Scholar]
- Ushakova, N.A.; Brodskii, E.S.; Kovalenko, A.A.; Bastrakov, A.I.; Kozlova, A.A.; Pavlov, D.S. Characteristics of lipid fractions of larvae of the black soldier fly Hermetia illucens. Dokl. Biochem. Biophys. 2016, 468, 209–212. [Google Scholar] [CrossRef]
- Surendra, K.C.; Olivier, R.; Tomberlin, J.K.; Jha, R.; Khanal, S.K. Bioconversion of organic wastes into biodiesel and animal feed via insect farming. Renew. Energy 2016, 98, 197–202. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Zheng, L.; Hou, Y.; Yang, S.; Yu, Z. Insect fat, a promising resource for biodiesel. J. Pet. Environ. Biotechnol. 2011, 2, 2–4. [Google Scholar]
- Kroeckel, S.; Harjes, A.G.E.; Roth, I.; Katz, H.; Wuertz, S.; Susenbeth, A.; Schulz, C. When a turbot catches a fly: Evaluation of a pre-pupae meal of the Black Soldier Fly (Hermetia illucens) as fish meal substitute—Growth performance and chitin degradation in juvenile turbot (Psetta maxima). Aquaculture 2012, 364–365, 345–352. [Google Scholar] [CrossRef]
- Caligiani, A.; Marseglia, A.; Leni, G.; Baldassarre, S.; Maistrello, L.; Dossena, A.; Sforza, S. Composition of black soldier fly prepupae and systematic approaches for extraction and fractionation of proteins, lipids and chitin. Food Res. Int. 2018, 105, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J.A. Nutritional value of the black soldier fly (Hermetia illucens L.) and its suitability as animal feed—A review. J. Insects Food Feed 2017, 3, 105–120. [Google Scholar] [CrossRef]
- Barroso, F.G.; Sánchez-Muros, M.J.; Segura, M.; Morote, E.; Torres, A.; Ramos, R.; Guil, J.-L. Insects as food: Enrichment of larvae of Hermetia illucens with omega 3 fatty acids by means of dietary modifications. J. Food Compos. Anal. 2017, 62, 8–13. [Google Scholar] [CrossRef]
- Meneguz, M.; Schiavone, A.; Gai, F.; Dama, A.; Lussiana, C.; Renna, M.; Gasco, L. Effect of rearing substrate on growth performance, waste reduction efficiency and chemical composition of black soldier fly (Hermetia illucens) larvae. J. Sci. Food Agric. 2018, 98, 5776–5784. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.Y.; Kutty, S.R.M.; Tan, C.K.; Tey, L.H. Comparative study on the effect of organic waste on lauric acid produced by Hermetia illucens larvae via bioconversion. Int. J. Eng. Sci. Technol. 2015, 8, 52–63. [Google Scholar]
- Nakatsuji, T.; Kao, M.C.; Fang, J.Y.; Zouboulis, C.C.; Zhang, L.; Gallo, R.L.; Huang, C.-M. Antimicrobial Property of Lauric Acid Against Propionibacterium Acnes: Its Therapeutic Potential for Inflammatory Acne Vulgaris. J. Investig. Dermatol. 2009, 129, 2480–2488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.M.; Lee, S.J.; Yu, H.; Park, J.Y.; Jung, H.S.; Kim, K.; Lee, C.J.; Chang, P.-S. Hydrophilic and lipophilic characteristics of non-fatty acid moieties: Significant factors affecting antibacterial activity of lauric acid esters. Food Sci. Biotechnol. 2018, 27, 401–409. [Google Scholar] [CrossRef]
- Zeiger, K.; Popp, J.; Becker, A.; Hankel, J.; Visscher, C.; Klein, G.; Meemken, D. Lauric acid as feed additive–An approach to reducing Campylobacter spp. in broiler meat. PLoS ONE 2017, 12, e0175693. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Hu, Y.; Xu, D.; Cai, K. Surface functionalization of titanium substrates with chitosan–lauric acid conjugate to enhance osteoblasts functions and inhibit bacteria adhesion. Colloids Surf. B Biointerfaces 2014, 119, 115–125. [Google Scholar] [CrossRef]
- Diclaro, J.W., II.; Kaufman, P.E. Black soldier fly Hermetia illucens linnaeus (insecta: Diptera: Stratiomyidae). EDIS 2009, 7, 1–4. [Google Scholar]
- Borrelli, L.; Varriale, L.; Dipineto, L.; Pace, A.; Menna, L.F.; Fioretti, A. Insect Derived Lauric Acid as Promising Alternative Strategy to Antibiotics in the Antimicrobial Resistance Scenario. Front. Microbiol. 2021, 12, 620798. [Google Scholar] [CrossRef]
- Dijkstra, A.J.; Van Opstal, M. The total degumming process. J. Am. Oil Chem. Soc. 1989, 66, 1002–1009. [Google Scholar] [CrossRef]
- Parsons, C.I. Fuller’s Earth and Its Application to the Bleaching of Oils. J. Am. Chem. Soc. 1907, 29, 598–605. [Google Scholar] [CrossRef] [Green Version]
- Beal, R.E.; Lancaster, E.B. Effect of agitation on selectivity in the hydrogenation of soybean oil. J. Am. Oil Chem. Soc. 1954, 31, 619–625. [Google Scholar] [CrossRef]
- Zielińska, A.; Nowak, I. Fatty acids in vegetable oils and their importance in cosmetic industry. CHEMIK Nauka-Tech.-Rynek 2014, 68, 103–110. [Google Scholar]
- Julita, U.; Suryani, Y.; Kinasih, I.; Yuliawati, A.; Cahyanto, T.; Maryeti, Y.; Permana, A.D.; Fitri, L.L. Growth performance and nutritional composition of black soldier fly, Hermetia illucens (L), (Diptera: Stratiomyidae) reared on horse and sheep manure. IOP Conf. Ser. Earth Environ. Sci. 2018, 187, 012071. [Google Scholar] [CrossRef]
- Ruhnke, I.; Normant, C.; Campbell, D.L.M.; Iqbal, Z.; Lee, C.; Hinch, G.N.; Roberts, J. Impact of on-range choice feeding with black soldier fly larvae (Hermetia illucens) on flock performance, egg quality, and range use of free-range laying hens. Anim. Nutr. 2018, 4, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Green, P.; Guy, R.; Hadgraft, J. In vitro and in vivo enhancement of skin permeation with oleic and lauric acids. Int. J. Pharm. 1988, 48, 103–111. [Google Scholar] [CrossRef]
- Dubois, V.; Breton, S.; Linder, M.; Fanni, J.; Parmentier, M. Fatty acid profiles of 80 vegetable oils with regard to their nutritional potential. Eur. J. Lipid Sci. Technol. 2007, 109, 710–732. [Google Scholar] [CrossRef]
- Almeida, C.; Rijo, P.; Rosado, C. Bioactive Compounds from Hermetia illucens Larvae as Natural Ingredients for Cosmetic Application. Biomolecules 2020, 10, 976. [Google Scholar] [CrossRef]
- Anzaku, A.A.; Akyala, J.I.; Juliet, A.; Obianuju, E.C. Antibacterial Activity of Lauric Acid on Some Selected Clinical Isolates. Ann. Clin. Lab. Res. 2017, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Sangduan, C. Skincare Product Containing Hermetia illucens Extract. U.S. Patent Application No 15/981,689, 13 September 2018. [Google Scholar]
- Manniello, M.D.; Moretta, A.; Salvia, R.; Scieuzo, C.; Lucchetti, D.; Vogel, H.; Sgambato, A.; Falabella, P. Insect antimicrobial peptides: Potential weapons to counteract the antibiotic resistance. Cell. Mol. Life Sci. 2021, 78, 4259–4282. [Google Scholar] [CrossRef]
- Moretta, A.; Salvia, R.; Scieuzo, C.; Di Somma, A.; Vogel, H.; Pucci, P.; Sgambato, A.; Wolff, M.; Falabella, P. A bioinformatic study of antimicrobial peptides identified in the Black Soldier Fly (BSF) Hermetia illucens (Diptera: Stratiomyidae). Sci. Rep. 2020, 10, 16875. [Google Scholar] [CrossRef] [PubMed]
- Moretta, A.; Scieuzo, C.; Petrone, A.M.; Salvia, R.; Manniello, M.D.; Franco, A.; Lucchetti, D.; Vassallo, A.; Vogel, H.; Sgambato, A.; et al. Antimicrobial Peptides: A New Hope in Biomedical and Pharmaceutical Fields. Front. Cell. Infect. Microbiol. 2021, 11, 668632. [Google Scholar] [CrossRef]
- Rahnamaeian, M.; Vilcinskas, A. Short antimicrobial peptides as cosmetic ingredients to deter dermatological pathogens. Appl. Microbiol. Biotechnol. 2015, 99, 8847–8855. [Google Scholar] [CrossRef] [Green Version]
- Dutta, P.K.; Dutta, J.; Tripathi, V.S. Chitin and Chitosan: Chemistry, Properties and Applications. J. Sci. Ind. Res. 2004, 63, 20–31. [Google Scholar]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Scieuzo, C.; Hahn, T.; Zibek, S.; Salvia, R.; Falabella, P. Insect Chitin-Based Nanomaterials for Innovative Cosmetics and Cosmeceuticals. Cosmetics 2021, 8, 40. [Google Scholar] [CrossRef]
- Hahn, T.; Tafi, E.; Paul, A.; Salvia, R.; Falabella, P.; Zibek, S. Current state of chitin purification and chitosan production from insects. J. Chem. Technol. Biotechnol. 2020, 95, 2775–2795. [Google Scholar] [CrossRef]
- Ermolaev, E.; Lalander, C.; Vinnerås, B. Greenhouse gas emissions from small-scale fly larvae composting with Hermetia illucens. Waste Manag. 2019, 96, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.; Hou, D.; Chen, J.; Nowar, E.E.; Li, Z.; Hu, R.; Tomberlin, J.K.; Yu, Z.; Li, Q.; Wang, S. Reducing greenhouse gas emissions and enhancing carbon and nitrogen conversion in food wastes by the black soldier fly. J. Environ. Manag. 2020, 260, 110066. [Google Scholar] [CrossRef] [PubMed]



| Lipid Yield (%/DW) | C 12:0 (%) | C 14:0 (%) | C 16:0 (%) | C18:1 n-9 (%) | |
|---|---|---|---|---|---|
| Hermetia illucens [46] | 7.20–42.60 | 23.90–61.87 | 3.85–10.35 | 5.78–20.42 | 4.27–28.80 |
| Musca domestica [47] | 21.70 ± 1.70 | 0.00 | 0.46 ± 0.01 | 5.10 ± 0.07 | 7.21 ± 0.08 |
| Tenebrio molitor [48] | 31.97 ± 1.60 | 0.00 | 4.45 ± 0.02 | 21.33 ± 0.13 | 35.83 ± 0.33 |
| Alphitobius diaperinus [49] | 26.25 ± 0.01 | 0.03 ± 0.04 | 0.77 ± 0.01 | 24.98 ± 0.05 | 28.97 ± 0.01 |
| Acheta domesticus [48] | 15.31 ± 0.18 | 0.1 ± 0.00 | 0.44 ± 0.00 | 22.65 ± 0.37 | 20.18 ± 0.02 |
| Gryllodes sigillatus [50] | 18.23 ± 0.70 | 0.1 ± 0.02 | 1.65 ± 0.12 | 23.5 ± 0.65 | 29.14 ± 1.50 (C18:1n9c + C18:1n9) |
| Gryllus assimilis [51] | 21.80 ± 2.65 | 0.10 ± 0.02 | 1.00 ± 0.00 | 33.10 ± 1.86 | 30.30 ± 0.27 |
| Coconut oil [52,53] | 33.00 | 44.10–51.00 | 13.10–18.50 | 7.50–10.50 | 5.00–8.20 |
| Commercial palm oil [54,56] | 45.00–55.00 | 0.20 | 1.10 | 44.00 | 39.20 |
| Commercial palm kernel oil [54,55] | 50.00 | 47.80 | 16.30 | 8.50 | 15.40 |
| Insect Species | Lipid Yield (%) | Extraction Methods |
|---|---|---|
| Hermetia illucens | 40.96 ± 0.93 (DW) | Soxhlet (Petroleum ether) [59] |
| 34.54 (WW) | Folch [60] | |
| 37.10 ± 1.10 (DW) | Soxhlet (Diethyl ether) [11] | |
| Musca domestica | 21.7 ± 1.7 (DW) | Soxhlet (Petroleum ether) [47] |
| 24.56 (DW) | Soxhlet (Petroleum ether) [61] | |
| Tenebrio molitor | 7.8 ± 0.4 (WW) | Aqueous [57] |
| 12.7 ± 2.4 (WW) | Soxhlet [57] | |
| 12.9 ± 0.2 (WW) | Folch [57] | |
| 25.5 ± 0.1 (WW) | Soxhlet (Hexane) [58] | |
| 24.3 ± 1.2 (WW) | Soxhlet (Petroleum ether) [58] | |
| 25.7 ± 0.3 (WW) | Soxhlet (Ethyl acetate) [58] | |
| 28.8 ± 5.9 (WW) | Soxhlet (Ethanol) [58] | |
| 23.7 ± 2.4 (WW) | TPP [58] | |
| 22.1 ± 0.6 (WW) | SC-C2 [58] | |
| Alphitobius diaperinus | 5.5 ± 1.0 (WW) | Aqueous [57] |
| 10.7 ± 0.5 (WW) | Soxhlet [57] | |
| 9.4 ± 1.0 (WW) | Folch [57] | |
| Acheta domesticus | 1.6 ± 0.1 (WW) | Aqueous [57] |
| 6.0 ± 0.3 (WW) | Soxhlet [57] | |
| 8.0 ± 1.1 (WW) | Folch [57] | |
| 14.6 ± 0.1 (WW) | Soxhlet (Hexane) [58] | |
| 14.7 ± 0.2 (WW) | Soxhlet (Petroleum ether) [58] | |
| 15.1 ± 0.3 (DW) | Soxhlet (Ethyl acetate) [58] | |
| 22.7 ± 2.9 (DW) | Soxhlet (Ethanol) [58] | |
| 19.3 ± 2.0 (DW) | TPP [58] | |
| 11.9 ± 1.4 (DW) | SC-CO2 [58] | |
| Gryllodes sigillatus | 18.23 ± 0.7 (DW) | Soxhlet (hexane) [50] |
| Gryllus assimilis | 21.80 ± 2.65 (DW) | Soxhlet (Petroleum ether) [51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco, A.; Salvia, R.; Scieuzo, C.; Schmitt, E.; Russo, A.; Falabella, P. Lipids from Insects in Cosmetics and for Personal Care Products. Insects 2022, 13, 41. https://doi.org/10.3390/insects13010041
Franco A, Salvia R, Scieuzo C, Schmitt E, Russo A, Falabella P. Lipids from Insects in Cosmetics and for Personal Care Products. Insects. 2022; 13(1):41. https://doi.org/10.3390/insects13010041
Chicago/Turabian StyleFranco, Antonio, Rosanna Salvia, Carmen Scieuzo, Eric Schmitt, Antonella Russo, and Patrizia Falabella. 2022. "Lipids from Insects in Cosmetics and for Personal Care Products" Insects 13, no. 1: 41. https://doi.org/10.3390/insects13010041
APA StyleFranco, A., Salvia, R., Scieuzo, C., Schmitt, E., Russo, A., & Falabella, P. (2022). Lipids from Insects in Cosmetics and for Personal Care Products. Insects, 13(1), 41. https://doi.org/10.3390/insects13010041