Consequences of Thermal Variation during Development and Transport on Flight and Low-Temperature Performance in False Codling Moth (Thaumatotibia leucotreta): Fine-Tuning Protocols for Improved Field Performance in a Sterile Insect Programme
Abstract
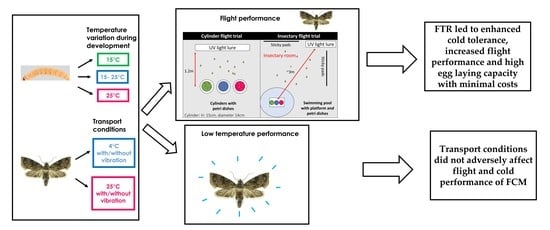
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Developmental Plasticity and Performance
2.1.1. Larval Acclimation Treatments on Adult Development and Performance
2.1.2. Life History
2.1.3. Low-Temperature Performance
2.1.4. Flight Performance
Morphometrics
2.2. Adult Transport Conditions and Performance
2.2.1. Simulating Transport Conditions
2.2.2. Chill Coma Recovery Time (CCRT) and Spontaneous Behaviour
2.2.3. Flight Performance
2.3. Statistical Analysis
2.3.1. Acclimation Treatments, Life History and Performance
Morphometrics
2.3.2. Flight Performance in Response to Adult Transport Conditions
3. Results
3.1. Larval Acclimation Treatment Effects on Adult Performance
3.1.1. Life History
3.1.2. Low-Temperature Performance
3.1.3. Flight Performance
Morphometrics
3.2. Adult Transport Conditions and Performance
3.2.1. Chill Coma Recovery Time (CCRT) and Spontaneous Behaviour
3.2.2. Flight Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EPPO. PQR. Database. European and Mediterranean Plant Protection Organization, Paris, France. 2022. Available online: https://gd.eppo.int/taxon/ARGPLE/distribution (accessed on 14 March 2022).
- EPPO. EPPO Member Countries. Available online: https://www.eppo.int/ABOUT_EPPO/eppo_members (accessed on 8 February 2022).
- Carpenter, J.; Bloem, S.; Hofmeyr, H. Area-Wide Control Tactics for the False Codling Moth Thaumatotibia Leucotreta in South Africa: A Potential Invasive Species, Area-Wide Control of Insect Pests; Springer: Dordrecht, The Netherlands, 2007; pp. 351–359. [Google Scholar]
- Hofmeyr, J.H.; Pringle, K.L. Resistance of false codling moth, Cryptophlebia leucotreta (Meyrick) (Lepidoptera: Tortricidae), to the chitin synthesis inhibitor, triflumuron. Afr. Entomol. 1998, 6, 373–375. [Google Scholar]
- Barnes, B.N.; Hofmeyr, J.H.; Groenewald, S.; Conlong, D.E.; Wohlfarter, M. The sterile insect technique in agricultural crops in South Africa: A metamorphosis… but will it fly? Afr. Entomol. 2015, 23, 1–18. [Google Scholar] [CrossRef]
- Boersma, N.; Boardman, L.; Gilbert, M.; Terblanche, J.S. Sex-dependent thermal history influences cold tolerance, longevity and fecundity in false codling moth Thaumatotibia leucotreta (Lepidoptera: T ortricidae). Agric. For. Entomol. 2018, 20, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Hofmeyr, J.H.; Carpenter, J.E.; Bloem, S.; Slabbert, J.P.; Hofmeyr, M.; Groenewald, S.S. Development of the sterile insect technique to suppress false codling moth Thaumatotibia leucotreta (Lepidoptera: Tortricidae) in citrus fruit: Research to implementation (Part 1). Afr. Entomol. 2015, 23, 180–186. [Google Scholar] [CrossRef]
- Klassen, W.; Curtis, C.S. History of the sterile insect technique. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 3–36. [Google Scholar]
- Sørensen, J.G.; Addison, M.F.; Terblanche, J.S. Mass-rearing of insects for pest management: Challenges, synergies and advances from evolutionary physiology. Crop Prot. 2012, 38, 87–94. [Google Scholar] [CrossRef]
- Vreysen, M.J.B.; Carpenter, J.E.; Marec, F. Improvement of the sterile insect technique for codling moth Cydia pomonella (Linnaeus) (Lepidoptera, Tortricidae) to facilitate expansion of field application. J. Appl. Entomol. 2010, 134, 165–181. [Google Scholar] [CrossRef]
- Debat, V.; Cornette, R.; Korol, A.B.; Nevo, E.; Soulet, D.; David, J.R. Multidimensional analysis of Drosophila wing variation in Evolution Canyon. J. Genet. 2008, 87, 407–419. [Google Scholar] [CrossRef]
- Frazier, M.R.; Harrison, J.F.; Kirkton, S.D.; Roberts, S.P. Cold rearing improves cold-flight performance in Drosophila via changes in wing morphology. J. Exp. Biol. 2008, 211, 2116–2122. [Google Scholar] [CrossRef] [Green Version]
- Shirai, Y. Factors influencing flight ability of male adults of the diamondback moth, Plutella xylostella, with special reference to temperature conditions during the larval stage. Appl. Entomol. Zool. 1993, 28, 291–301. [Google Scholar] [CrossRef] [Green Version]
- Debat, V.; Bégin, M.; Legout, H.; David, J.R. Allometric and no allometric components of Drosophila wing shape respond differently to developmental temperature. Evolution 2003, 57, 2773–2784. [Google Scholar] [CrossRef]
- Kristensen, T.N.; Loeschcke, V.; Hoffmann, A.A. Linking inbreeding effects in captive populations with fitness in the wild: Release of replicated Drosophila melanogaster lines under different temperatures. Conserv. Biol. 2008, 22, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Chidawanyika, F.; Terblanche, J.S. Rapid Thermal responses and thermal tolerance in adult codling moth Cydia pomonella (Lepidoptera: Tortricidae). J. Insect Physiol. 2011, 57, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Judd, G.J.; Gardiner, M.G. Temperature, irradiation and delivery as factors affecting spring-time flight activity and recapture of mass-reared male codling moths released by the Okanagan-Kootenay sterile insect programme. J. Entomol. Soc. Br. Columbia 2006, 103, 19–32. [Google Scholar]
- Judd, G.J.; Arthur, S.; Deglow, K.; Gardiner, M.G. Operational Mark-Release-Recapture Field Tests Comparing Competitiveness of Wild and Differentially Mass-Reared Codling Moths from the Okanagan-Kootenay Sterile Insect Program. Can. Entomol. 2011, 143, 300–316. [Google Scholar] [CrossRef]
- Matveev, E.; Kwon, J.J.; Judd, G.J.R.; Evenden, M.L. The effect of cold storage of mass-reared codling moths (Lepidoptera: Tortricidae) on subsequent flight capacity. Can. Entomol. 2017, 149, 391–398. [Google Scholar] [CrossRef]
- Boersma, N.; Carpenter, J.E. Influence of holding temperature and irradiation on field performance of mass reared Thaumatotibia leucotreta (Lepidoptera: Tortricidae). Fla. Entomol. 2016, 99 (Suppl. S1), 215–221. [Google Scholar] [CrossRef]
- Terblanche, J.S.; Mitchell, K.A.; Uys, W.; Short, C.; Boardman, L. Thermal limits to survival and activity in two life stages of false codling moth Thaumatotibia leucotreta (Lepidoptera, Tortricidae). Physiol. Entomol. 2017, 42, 379–388. [Google Scholar] [CrossRef]
- Stotter, R.L.; Terblanche, J.S. Low-temperature tolerance of false codling moth Thaumatotibia leucotreta (Meyrick) (Lepidoptera: Tortricidae) in South Africa. J. Therm. Biol. 2009, 34, 320–325. [Google Scholar]
- Clark, M.S.; Worland, M.R. How insects survive the cold: Molecular mechanisms—a review. J. Comp. Physiol. B. 2008, 178, 917–933. [Google Scholar] [CrossRef] [Green Version]
- Nepgen, E.S.; Hill, M.P.; Moore, S.D. The effect of long-distance transportation on the fitness of irradiated false codling Moth (Lepidoptera: Tortricidae) for use in a sterile insect release program. J. Econ. Entomol. 2015, 108, 2610–2619. [Google Scholar] [CrossRef] [PubMed]
- Simmons, G.S.; Suckling, D.M.; Carpenter, J.E.; Addison, M.F.; Dyck, V.A.; Vreysen, M.J.B. Improved quality management to enhance the efficacy of the sterile insect technique for lepidopteran pests. J. Appl. Entomol. 2010, 134, 261–273. [Google Scholar] [CrossRef]
- Benelli, M.; Mainali, B.; Taylor, P.W.; Rempoulakis, P. Reduced quality of sterile Queensland fruit fly following post-production stress from hypoxia, irradiation and vibration. J. Pest Sci. 2021, 94, 473–485. [Google Scholar] [CrossRef]
- Fischer, K.; Kölzow, N.; Höltje, H.; Karl, I. Assay conditions in laboratory experiments: Is the use of constant rather than fluctuating temperatures justified when investigating temperature-induced plasticity? Oecologia 2011, 166, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Colinet, H.; Sinclair, B.J.; Vernon, P.; Renault, D. Insects in fluctuating thermal environments. Annu. Rev. Entomol. 2015, 60, 123–140. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, B.J.; Sørensen, J.G.; Terblanche, J.S. Harnessing thermal plasticity to enhance the performance of mass-reared insects: Opportunities and challenges. Bull. Entomol. Res. 2022; in press. [Google Scholar]
- Diallo, S.; Seck, M.T.; Rayaissé, J.B.; Fall, A.G.; Bassene, M.D.; Sall, B.; Sanon, A.; Vreysen, M.J.; Takac, P.; Parker, A.G.; et al. Chilling, irradiation and transport of male Glossina palpalis gambiensis pupae: Effect on the emergence, flight ability and survival. PLoS ONE 2019, 14, e0216802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Xi, Z.; Li, Y.; Wang, X.; Yamada, H.; Qiu, J.; Liang, Y.; Zhang, M.; Wu, Y.; Zheng, X. Toward implementation of combined incompatible and sterile insect techniques for mosquito control: Optimized chilling conditions for handling Aedes albopictus male adults prior to release. PLoS Negl. Trop. Dis. 2020, 14, e0008561. [Google Scholar] [CrossRef] [PubMed]
- Rull, J.; Birke, A.; Ortega, R.; Montoya, P.; López, L. Quantity and safety vs. quality and performance: Conflicting interests during mass rearing and transport affect the efficiency of sterile insect technique programs. Entomol. Exp. Et Appl. 2012, 142, 78–86. [Google Scholar] [CrossRef]
- Pagabeleguem, S.; Seck, M.T.; Sall, B.; Vreysen, M.J.; Gimonneau, G.; Fall, A.G.; Bassene, M.; Sidibé, I.; Rayaissé, J.B.; Belem, A.M.; et al. Long distance transport of irradiated male Glossina palpalis gambiensis pupae and its impact on sterile male yield. Parasites Vectors 2015, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Moore, S.D.; Richards, G.I.; Chambers, C.; Hendry, D. An improved larval diet for commercial mass rearing of the false codling moth, Thaumatotibia leucotreta (Meyrick) (Lepidoptera: Tortricidae). Afr. Entomol. 2014, 22, 216–219. [Google Scholar] [CrossRef]
- Boardman, L.; Grout, T.G.; Terblanche, J.S. False codling moth Thaumatotibia leucotreta (Lepidoptera, Tortricidae) larvae are chill-susceptible. Insect Sci. 2012, 19, 315–328. [Google Scholar] [CrossRef]
- Ransberry, V.E.; MacMillan, H.A.; Sinclair, B.J. The relationship between chill-coma onset and recovery at the extremes of the thermal window of Drosophila melanogaster. Physiol. Biochem. Zool. 2011, 84, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.E.; Blomefield, T.; Vreysen, M.J.B. A flight cylinder bioassay as a simple, effective quality control test for Cydia pomonella. J. Appl. Entomol. 2012, 136, 711–720. [Google Scholar] [CrossRef]
- Shelly, T.E.; Edu, J.; Nishimoto, J. Chilling and flight ability and mating competitiveness of sterile males of the Mediterranean fruit fly. J. Appl. Entomol. 2013, 137, 11–18. [Google Scholar] [CrossRef]
- Seck, M.T.; Pagabeleguem, S.; Bassene, M.D.; Fall, A.G.; Diouf, T.A.; Sall, B.; Vreysen, M.J.; Rayaisse, J.B.; Takac, P.; Sidibe, I.; et al. Quality of sterile male tsetse after long distance transport as chilled, irradiated pupae. PLoS Negl. Trop. Dis. 2015, 9, e0004229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visser, A. Determining the Quality of Mass Reared Male Codling Moth, Cydia pomonella (Lepidoptera: Tortricidae), by Assessing Flight Performance under Laboratory, Semi-Field and Field Conditions. Masters Thesis, University of Stellenbosch, Stellenbosch, South Africa, 2016. [Google Scholar]
- Morland, G. The Morphology and Ecology of the Carob Moth (Ectomyelois ceratoniae) (Zeller) in Citrus Orchards of the Western Cape, South Africa. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2015. [Google Scholar]
- Esterhuizen, N.; Clusella-Trullas, S.; Van Daalen, C.E.; Schoombie, R.E.; Boardman, L.; Terblanche, J.S. Effects of within-generation thermal history on the flight performance of Ceratitis capitata: Colder is better. J. Exp. Biol. 2014, 217, 3545–3556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; ISBN 0-387-95457-0. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019; Available online: https://socialsciences.mcmaster.ca/jfox/Books/Companion/ (accessed on 14 March 2022).
- Length, R. Emmeans: Estimated Marginal Means, Aka Least-Squares Means, R package Version 1.5.1. 2020. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 14 March 2022).
- Kleiber, C.; Zeileis, A. Applied Econometrics with R; Springer: New York, NY, USA, 2008; Available online: https://CRAN.R-project.org/package=AER (accessed on 14 March 2022)ISBN 978-0-387-77316-2.
- Harrell, F.E.; With Contributions from Charles Dupont and Many Others. Hmisc: Harrell Miscellaneous, R Package Version 4.4-1; 2020. Available online: https://CRAN.R-project.org/package=Hmisc (accessed on 14 March 2022).
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix, Version 0.84. 2017. Available online: https://github.com/taiyun/corrplot (accessed on 14 March 2022).
- Chown, S.L.; Chown, S.; Nicolson, S. Insect Physiological Ecology: Mechanisms and Patterns; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Dell, A.I.; Pawar, S.; Savage, V.M. Systematic variation in the temperature dependence of physiological and ecological traits. Proc. Natl. Acad. Sci. USA 2011, 108, 10591–10596. [Google Scholar] [CrossRef] [Green Version]
- Terblanche, J.S. Physiological performance of field-released insects. Curr. Opin. Insect Sci. 2014, 4, 60–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colinet, H.; Renault, D.; Hance, T.; Vernon, P. The impact of fluctuating thermal regimes on the survival of a cold-exposed parasitic wasp, Aphidius colemani. Physiol. Entomol. 2006, 31, 234–240. [Google Scholar] [CrossRef]
- Colinet, H.; Rinehart, J.P.; Yocum, G.D.; Greenlee, K.J. Mechanisms underpinning the beneficial effects of fluctuating thermal regimes in insect cold tolerance. J. Exp. Biol. 2018, 221, jeb164806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renault, D.; Nedved, O.; Hervant, F.; Vernon, P. The importance of fluctuating thermal regimes for repairing chill injuries in the tropical beetle Alphitobius diaperinus (Coleoptera: Tenebrionidae) during exposure to low temperature. Physiol. Entomol. 2004, 29, 139–145. [Google Scholar] [CrossRef]
- Mahi, H.; Rasekh, A.; Michaud, J.P.; Shishehbor, P. Biology of Lysiphlebus fabarum following cold storage of larvae and pupae. Entomol. Exp. Et Appl. 2014, 153, 10–19. [Google Scholar] [CrossRef]
- Torson, A.S.; Yocum, G.D.; Rinehart, J.P.; Nash, S.A.; Kvidera, K.M.; Bowsher, J.H. Physiological responses to fluctuating temperatures are characterized by distinct transcriptional profiles in a solitary bee. J. Exp. Biol. 2017, 220, 3372–3380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grumiaux, C.; Andersen, M.K.; Colinet, H.; Overgaard, J. Fluctuating thermal regime preserves physiological homeostasis and reproductive capacity in Drosophila suzukii. J. Insect Physiol. 2019, 113, 33–41. [Google Scholar] [CrossRef]
- Duffy, G.A.; Coetzee, B.W.; Janion-Scheepers, C.; Chown, S.L. Microclimate-based macrophysiology: Implications for insects in a warming world. Curr. Opin. Insect Sci. 2015, 11, 84–89. [Google Scholar] [CrossRef]
- Chown, S.L.; Terblanche, J.S. Physiological diversity in insects: Ecological and evolutionary contexts. Adv. Insect Physiol. 2006, 33, 50–152. [Google Scholar]
- Slotsbo, S.; Schou, M.F.; Kristensen, T.N.; Loeschcke, V.; Sørensen, J.G. Reversibility of developmental heat and cold plasticity is asymmetric and has long-lasting consequences for adult thermal tolerance. J. Exp. Biol. 2016, 219, 2726–2732. [Google Scholar] [CrossRef] [Green Version]
- Suckling, D.M.; Conlong, D.E.; Carpenter, J.E.; Bloem, K.A.; Rendon, P.; Vreysen, M.J.B. Global range expansion of pest Lepidoptera requires socially acceptable solutions. Biol. Invasions 2017, 19, 1107–1119. [Google Scholar] [CrossRef] [Green Version]
- Alphey, L.S. Engineering insects for the sterile insect technique. In Area-Wide Control of Insect Pests; Springer: Dordrecht, The Netherland, 2007; pp. 51–60. [Google Scholar]
- Hight, S.D.; Carpenter, J.E.; Bloem, S.; Bloem, K.A. Developing a sterile insect release program for Cactoblastis cactorum (Berg) (Lepidoptera: Pyralidae): Effective overflooding ratios and release-recapture field studies. Environ. Entomol. 2005, 34, 850–856. [Google Scholar] [CrossRef] [Green Version]
- Boersma, N.; Boardman, L.; Gilbert, M.; Terblanche, J.S. Cold treatment enhances low-temperature flight performance in false codling moth, Thaumatotibia leucotreta (Lepidoptera: Tortricidae). Agric. For. Entomol. 2019, 21, 243–251. [Google Scholar] [CrossRef]
- Komai, F. A taxonomic review of the genus Grapholita and allied genera (Lepidoptera: Tortricidae) in the Palaearctic region. Entomol. Scand. Suppl. 1999, 55, 1–226. [Google Scholar]





| Chill Coma Recovery Time (CCRT) | χ2 | df | p-Value | |
|---|---|---|---|---|
| Treatment | 16.6740 | 3 | <0.002 | |
| Sex | 0.8560 | 1 | 0.3549 | |
| Treatment × Sex | 35.6400 | 3 | <0.0001 | |
| Spontaneous behaviour | χ2 | df | p-value | |
| Treatment | 2.4510 | 3 | 0.4842 | |
| Sex | 0.2414 | 1 | 0.6232 | |
| Treatment × Sex | 1.6119 | 3 | 0.6567 | |
| Flight performance: Cylinder | F | df | Resid df | p-value |
| Treatment | 0.4242 | 3 | 20 | 0.7383 |
| Sex | 7.6522 | 1 | 19 | <0.02 |
| Treatment × Sex | 0.3505 | 3 | 16 | 0.7894 |
| Flight performance: Insectary | F | df | Resid df | p-value |
| Treatment | 7.62 | 3 | 20 | <0.003 |
| Sex | 5.7093 | 1 | 19 | <0.03 |
| Treatment × Sex | 1.8443 | 3 | 16 | 0.1798 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huisamen, E.J.; Karsten, M.; Terblanche, J.S. Consequences of Thermal Variation during Development and Transport on Flight and Low-Temperature Performance in False Codling Moth (Thaumatotibia leucotreta): Fine-Tuning Protocols for Improved Field Performance in a Sterile Insect Programme. Insects 2022, 13, 315. https://doi.org/10.3390/insects13040315
Huisamen EJ, Karsten M, Terblanche JS. Consequences of Thermal Variation during Development and Transport on Flight and Low-Temperature Performance in False Codling Moth (Thaumatotibia leucotreta): Fine-Tuning Protocols for Improved Field Performance in a Sterile Insect Programme. Insects. 2022; 13(4):315. https://doi.org/10.3390/insects13040315
Chicago/Turabian StyleHuisamen, Elizabeth J., Minette Karsten, and John S. Terblanche. 2022. "Consequences of Thermal Variation during Development and Transport on Flight and Low-Temperature Performance in False Codling Moth (Thaumatotibia leucotreta): Fine-Tuning Protocols for Improved Field Performance in a Sterile Insect Programme" Insects 13, no. 4: 315. https://doi.org/10.3390/insects13040315
APA StyleHuisamen, E. J., Karsten, M., & Terblanche, J. S. (2022). Consequences of Thermal Variation during Development and Transport on Flight and Low-Temperature Performance in False Codling Moth (Thaumatotibia leucotreta): Fine-Tuning Protocols for Improved Field Performance in a Sterile Insect Programme. Insects, 13(4), 315. https://doi.org/10.3390/insects13040315