Natural Repellents as a Method of Preventing Ant Damage to Microirrigation Systems
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
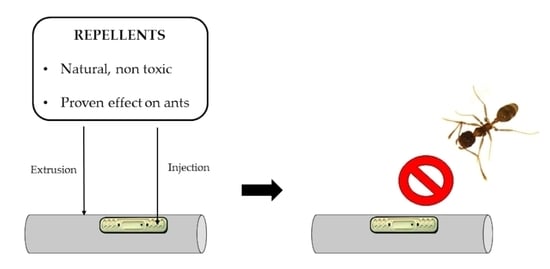
2.1. Repellents
2.2. Experimental Design
2.2.1. Olfactory Testing
2.2.2. Field Trials
2.3. Data Analyses
3. Results
3.1. Olfactory Testing
3.2. Field Trials
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hölldobler, B.; Wilson, E.O. The Ants; Springer: Berlin/Heidelberg, Germany, 1990. [Google Scholar]
- Guénard, B. An overview of the species and ecological diversity of ants. In eLS; John Wiley & Sons, Ltd.: Chichester, UK, 2013; pp. 1–10. ISBN 9780470015902. [Google Scholar]
- Yoo, H.J.S.; Kizner, M.C.; Holway, D.A. Ecological effects of multi-species, ant-hemipteran mutualisms in citrus. Ecol. Entomol. 2013, 38, 505–514. [Google Scholar] [CrossRef]
- Calabuig, A.; Garcia-Marí, F.; Pekas, A. Ants in citrus: Impact on the abundance, species richness, diversity and community structure of predators and parasitoids. Agric. Ecosyst. Environ. 2015, 213, 178–185. [Google Scholar] [CrossRef]
- Feng, D.D.; Michaud, J.P.; Li, P.; Zhou, Z.S.; Xu, Z.F. The native ant, Tapinoma melanocephalum, improves the survival of an invasive mealybug, Phenacoccus solenopsis, by defending it from parasitoids. Sci. Rep. 2015, 5, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Way, M.J.; Khoo, K.C. Role of ants in pest management. Annu. Rev. Entomol. 1992, 37, 479–503. [Google Scholar] [CrossRef]
- Styrsky, J.D.; Eubanks, M.D. Ecological consequences of interactions between ants and honeydew-producing insects. Proc. R. Soc. B Biol. Sci. 2007, 274, 151–164. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, J.A.; Carrasco-Ortiz, A.; López-Gallego, E.; Ramírez-Soria, M.J.; La Spina, M. Ants reduce fruit damage caused by psyllids in Mediterranean pear orchards. Pest Manag. Sci. 2021, 77, 1886–1892. [Google Scholar] [CrossRef]
- Sanchez, J.A.; Carrasco-Ortiz, A.; López-Gallego, E.; La-Spina, M. Ants (Hymenoptera: Formicidae) reduce the density of Cacopsylla pyri (Linnaeus, 1761) in Mediterranean pear orchards. Myrmecol. News 2020, 30, 93–102. [Google Scholar]
- Brown, J.H.; Reichman, O.J.; Davidson, D.W. Granivory in desert ecosystems. Annu. Rev. Ecol. Syst. 1979, 10, 201–227. [Google Scholar] [CrossRef]
- Blanton, C.M.; Ewel, J.J. Leaf-cutting ant herbivory in successional and agricultural tropical ecosystems. Ecol. Soc. Am. 1985, 66, 861–869. [Google Scholar] [CrossRef] [Green Version]
- Montoya-Lerma, J.; Giraldo-Echeverri, C.; Armbrecht, I.; Farji-Brener, A.; Calle, Z. Leaf-cutting ants revisited: Towards rational management and control. Int. J. Pest Manag. 2012, 58, 225–247. [Google Scholar] [CrossRef]
- Jetter, K.M.; Hamilton, J.; Klotz, J.H. Eradication costs calculated: Red imported fire ants threaten agriculture, wildlife and homes. Calif. Agric. 2002, 56, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Ota, A.K. Wireworm damage to polyethylene tubing used in a drip irrigation system. J. Econ. Entomol. 1973, 66, 824–825. [Google Scholar] [CrossRef]
- Ota, A.K.; Chang, V.C.S. Insect-Related Problems in Drip-Irrigation Systems; Association of Hawaiian Sugar Technologists: Wailuku, HI, USA, 1973; pp. 56–58. [Google Scholar]
- Chang, V.C.S.; Ota, A.K. Fire ant damage to polyethylene tubing used in drip irrigation systems. J. Econ. Entomol. 1976, 69, 447–450. [Google Scholar] [CrossRef]
- Stansly, P.; Pitts, D. Pest damage to micro-irrigation tubing: Causes and prevention. Proc. Fla. State Hortic. Soc. 1990, 103, 137–139. [Google Scholar]
- Boman, B.J. Ant impairment of Florida microirrigation systems. Appl. Eng. Agric. 2001, 17, 797–802. [Google Scholar] [CrossRef]
- Haney, P. A different approach to the Argentine ant problem. Citrograph 1984, 69, 140–146. [Google Scholar]
- Boman, B.; Ontermaa, E. Citrus microsprinkler clogging: Costs, causes, and cures. Proc. Fla. State Hortic. Soc. 1994, 107, 39–47. [Google Scholar]
- Boman, B.J. Effects of orifice size on microsprinkler clogging rates. Appl. Eng. Agric. 1995, 11, 839–843. [Google Scholar] [CrossRef]
- Boman, B.J.; Bullock, R.C.; Parsons, M.L. Ant damage to microsprinkler pulsator assemblies. Appl. Eng. Agric. 1995, 11, 835–837. [Google Scholar] [CrossRef]
- INE (Instituto Nacional de Estadística). Encuesta Sobre el Uso del Agua en el Sector Agrario. Available online: https://www.ine.es/prensa/euasa_2018.pdf (accessed on 1 July 2021).
- Eurostat (Statistical Office of the European Union) Database. Available online: https://ec.europa.eu/eurostat/data/database (accessed on 1 July 2021).
- Chang, V.C.S.; Ota, A.K.; Sanders, D. Parallel ridge barrier to control ant damage to orifices of drip irrigation tubes. J. Econ. Entomol. 1980, 73, 403–406. [Google Scholar] [CrossRef]
- Boman, B.J.; Parsons, L.R. Microsprinkler experiences in Florida citrus. Appl. Eng. Agric. 1999, 15, 465–475. [Google Scholar] [CrossRef]
- Moran, S.; Keidar, C.; Wolf, Y. Protecting polyethylene irrigation pipes against damage caused by woodpeckers. In Proceedings of the 9th Vertebrate Pest Conference, Fresno, CA, USA, 4–6 March 1980; pp. 43–48. [Google Scholar]
- Boman, B.J.; Bullock, R.C. Damage to microsprinkler riser assemblies from Selenisa sueroides caterpillars. Appl. Eng. Agric. 1994, 10, 221–223. [Google Scholar] [CrossRef]
- Sorensen, R.B.; Nuti, R.C.; Lamb, M.C. Rodent management for surface drip irrigation tubing in corn, cotton and peanut. Peanut Sci. 2007, 34, 32–37. [Google Scholar] [CrossRef]
- Hansen, S.; Jacob, J. Screening repellents for the management of rodent damage to subsurface drip irrigation systems. In Proceedings of the 6th Young Scientist Meeting, Quedlinburg, Germany, 27–29 November 2013; p. 20. [Google Scholar]
- Kain, P.; Boyle, S.M.; Tharadra, S.K.; Guda, T.; Pham, C.; Dahanukar, A.; Ray, A. Odour receptors and neurons for DEET and new insect repellents. Nature 2013, 502, 507–512. [Google Scholar] [CrossRef] [Green Version]
- Islam, J.; Zaman, K.; Tyagi, V.; Duarah, S.; Dhiman, S.; Chattopadhyay, P. Protection against mosquito vectors Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus using a novel insect repellent, ethyl anthranilate. Acta Trop. 2017, 174, 56–63. [Google Scholar] [CrossRef]
- Chen, S.; Chen, H.; Xu, Y. Safe chemical repellents to prevent the spread of invasive ants. Pest Manag. Sci. 2019, 75, 821–827. [Google Scholar] [CrossRef]
- Wang, K.; Tang, L.; Zhang, N.; Zhou, Y.; Li, W.; Li, H.; Cheng, D.; Zhang, Z. Repellent and fumigant activities of Eucalyptus globulus and Artemisia carvifolia essential oils against Solenopsis invicta. Bull. Insectol. 2014, 67, 207–211. [Google Scholar]
- Theis, N. Fragrance of Canada thistle (Cirsium arvense) attracts both floral herbivores and pollinators. J. Chem. Ecol. 2006, 32, 917–927. [Google Scholar] [CrossRef]
- Pattrick, J.G.; Shepherd, T.; Hoppitt, W.; Plowman, N.S.; Willmer, P. A dual function for 4-methoxybenzaldehyde in Petasites fragrans? Pollinator-attractant and ant-repellent. Arthropod. Plant. Interact. 2017, 11, 623–627. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.S.; Liu, J.Y.; Lin, C.Y.; Hsui, Y.R.; Lu, M.C.; Wu, W.J.; Chang, S.T. Terminating red imported fire ants using Cinnamomum osmophloeum leaf essential oil. Bioresour. Technol. 2008, 99, 889–893. [Google Scholar] [CrossRef]
- Huang, C.; Fu, J.; Liu, Y.; Cheng, D.; Zhang, Z.-X. The insecticidal and repellent activity of soil containing cinnamon leaf debris against Red Imported Fire Ant workers. Sociobiology 2015, 62, 46–51. [Google Scholar]
- Wiltz, B.A.; Suiter, D.R.; Gardner, W.A. Deterrency and toxicity of essential oils to Argentine and Red Imported Fire Ants (Hymenoptera: Formicidae). J. Entomol. Sci. 2007, 42, 239–249. [Google Scholar] [CrossRef]
- Davies, T.G.E.; Field, L.M.; Usherwood, P.N.R.; Williamson, M.S. DDT, pyrethrins, pyrethroids and insect sodium channels. IUBMB Life 2007, 59, 151–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsuda, Y. Progress and future of pyrethroids. Top. Curr. Chem. 2012, 314, 1–30. [Google Scholar]
- Islam, J.; Zaman, K.; Duarah, S.; Raju, P.S.; Chattopadhyay, P. Mosquito repellents: An insight into the chronological perspectives and novel discoveries. Acta Trop. 2017, 167, 216–230. [Google Scholar] [CrossRef]
- Gómez, C.; Espadaler, X. Variabilidad en la respuesta de Pheidole pallidula (Nyl.) como dispersante de semillas de especies del género Euphorbia L. Sci. Gerund. 1995, 21, 49–57. [Google Scholar]
- Campos, M.; Fernández, L.; Ruano, F.; Cotes, B.; Cárdenas, M.; Castro, J. Short term response of ants to the removal of ground cover in organic olive orchards. Eur. J. Entomol. 2011, 108, 417–423. [Google Scholar] [CrossRef] [Green Version]
- Akol, A.M.; Njagi, P.G.N.; Sithanantham, S.; Mueke, J.M. Effects of two neem insecticide formulations on the attractiveness, acceptability and suitability of diamondback moth larvae to the parasitoid, Diadegma mollipla (Holmgren) (Hym., Ichneumonidae). J. Appl. Entomol. 2003, 127, 325–331. [Google Scholar] [CrossRef] [Green Version]
- Saad, K.A.; Roff, M.; Hallett, R.H.; Idris, A.B. Aphid-induced defences in chilli affect preferences of the whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). Sci. Rep. 2015, 5, 13697. [Google Scholar] [CrossRef] [Green Version]
- Junker, R.R.; Blüthgen, N. Floral scents repel potentially nectar-thieving ants. Evol. Ecol. Res. 2008, 10, 295–308. [Google Scholar]
- Vaello, T.; Casas, J.L.; Pineda, A.; De Alfonso, I.; Marcos-García, M.Á. Olfactory response of the predatory bug Orius laevigatus (Hemiptera: Anthocoridae) to the aggregation pheromone of its prey, Frankliniella occidentalis (Thysanoptera: Thripidae). Environ. Entomol. 2017, 46, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Harbi, A.; de Pedro, L.; Ferrara, F.A.A.; Tormos, J.; Chermiti, B.; Beitia, F.; Sabater-Muñoz, B. Diachasmimorpha longicaudata parasitism response to medfly host fruit and fruit infestation age. Insects 2019, 10, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Pedro, L.; Harbi, A.; Tormos, J.; Sabater-Muñoz, B.; Beitia, F. A minor role of host fruit on the parasitic performance of Aganaspis daci (Hymenoptera: Figitidae) on medfly larvae. Insects 2021, 12, 345. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.D.; Acosta, F.J.; Ruiz, E. Claves Para la Identificación de la Fauna española. Las Subfamilias y Géneros de las Hormigas ibéricas; Universidad Complutense: Madrid, Spain, 1985. [Google Scholar]
- Lebas, C.; Galkowski, C.; Blatrix, R.; Wegnez, P. Guía de Campo de las Hormigas de Europa Occidental; Omega: Barcelona, Spain, 2016. [Google Scholar]
- R-Development-Core-Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistic with S; Statistics and Computing Book Series; Springer: New York, NY, USA, 2002. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [Green Version]
- Krause, C.; Ray, A. Conservation of olfactory avoidance in Drosophila species and identification of repellents for Drosophila suzukii. Sci. Rep. 2015, 5, 11527. [Google Scholar] [CrossRef]
- Api, A.M.; Belsito, D.; Bhatia, S.; Bruze, M.; Calow, P.; Dagli, M.L.; Dekant, W.; Fryer, A.D.; Kromidas, L.; La Cava, S.; et al. RIFM fragrance ingredient safety assessment, ethyl anthranilate, CAS registry number 87-25-2. Food Chem. Toxicol. 2015, 82, S97–S104. [Google Scholar] [CrossRef]
- Shumake, S.A.; Sterner, R.T.; Gaddis, S.E. Repellents to reduce cable gnawing by wild Norway rats. J. Wildl. Manage. 2000, 64, 1009–1013. [Google Scholar] [CrossRef]
- Jalenques, B. Environment friendly animal repellent masterbatches. In Proceedings of the 12th International Plastics Additives and Modifiers Conference, Cologne, Germany, 17–18 October 2006; p. 19. [Google Scholar]
- Etemad, N.; Bahramian, N.; Yosefypour, M.R. Pipelines safety improvement through controlling rodents. In Proceedings of the International Gas Union Research Conference (IGRC), Copenhagen, Denmark, 17–19 September 2014; Volume 1, pp. 2052–2059. [Google Scholar]
- Dethier, V.G.; Browne, B.L.; Smith, C.N. The designation of chemicals in terms of the responses they elicit from insects. J. Econ. Entomol. 1960, 53, 134–136. [Google Scholar] [CrossRef]
- White, G.B.; Moore, S.J. Terminology of insect repellents. In Insect Repellents Handbook; Debboun, M., Frances, S.P., Strickman, D.A., Eds.; CRC Press LLC: Boca Raton, FL, USA, 2015; pp. 3–30. [Google Scholar]
- Frouz, J.; Jilková, V. The effect of ants on soil properties and processes (Hymenoptera: Formicidae). Myrmecol. News 2008, 11, 191–199. [Google Scholar]
- Farji-Brener, A.G.; Werenkraut, V. The effects of ant nests on soil fertility and plant performance: A meta-analysis. J. Anim. Ecol. 2017, 86, 866–877. [Google Scholar] [CrossRef]
- Fischer, C.; Türke, M. Seed preferences by rodents in the agri-environment and implications for biological weed control. Ecol. Evol. 2016, 6, 5796–5807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, C.; Gayer, C.; Kurucz, K.; Riesch, F.; Tscharntke, T.; Batáry, P. Ecosystem services and disservices provided by small rodents in arable fields: Effects of local and landscape management. J. Appl. Ecol. 2018, 55, 548–558. [Google Scholar] [CrossRef] [Green Version]
- Rey Benayas, J.M.; Meltzer, J.; De Las Heras-Bravo, D.; Cayuela, L. Potential of pest regulation by insectivorous birds in Mediterranean woody crops. PLoS ONE 2017, 12, e0180702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamm, F.R. Advantages and Disadvantages of Subsurface Drip Irrigation. In Proceedings of the International Meeting on Advances in Drip/Micro Irrigation, Puerto de La Cruz, Spain, 2–5 December 2002; Santana Ojeda, J.L., Rodrigo López, J., Suárez Sánchez, C.L., Hernández Abreu, J.M., Marrero Domínguez, A., Eds.; INIA: Madrid, Spain, 2002; pp. 1–13. [Google Scholar]
- Salvador, R.; Aragüés, R. Estado de la cuestión del riego por goteo enterrado: Diseño, manejo, mantenimiento y control de la salinidad del suelo. ITEA Inf. Tec. Econ. Agrar. 2013, 109, 395–407. [Google Scholar] [CrossRef]
- Sorensen, R.B. Removing peanut foliage adjacent shallow subsurface drip laterals to reduce rodent damage. Crop. Forage Turfgrass Manag. 2019, 5, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Moran, S. Distribution and characteristics of the damage of the Syrian woodpecker, Dendrocopos syriacus (Hemp. & Ehr.) (Aves: Picidae), in polyethylene irrigation pipes in fruit orchards. Phytoparasitica 1977, 5, 127–139. [Google Scholar]
- Moran, S. Damage by vertebrates to plastic irrigation pipes in Israel. Phytoparasitica 1981, 9, 211–216. [Google Scholar] [CrossRef]
- Barnea, A.; Yom-Tov, Y. A method to deter Syrian woodpeckers from drilling holes in plastic irrigation pipes. Crop Prot. 1984, 3, 35–39. [Google Scholar] [CrossRef]
- Seifert, B. The ant genus Cardiocondyla (Insecta: Hymenoptera: Formicidae)—A taxonomic revision of the C. elegans, C. bulgarica, C. batesii, C. nuda, C. shuckardi, C. stambuloffii, C. wroughtonii, C. emeryi, and C. minutior species groups. Ann. Des Nat. Mus. Wien. Ser. B Für Bot. Und Zool. 2003, 104B, 203–338. [Google Scholar]
- Sharaf, M.R.; Aldawood, A.S.; Taylor, B. The formicine ant genus Plagiolepis Mayr (Hymenoptera: Formicidae) in the Arabian Peninsula, with description of two new species. Trans. Am. Entomol. Soc. 2011, 137, 203–215. [Google Scholar] [CrossRef]









| Type of Test | Comparison | χ2 | Df | p |
|---|---|---|---|---|
| Compounds vs. Control (Liquid) | CEO vs. Control | 16.90 | 1 | <0.001 |
| CIT vs. Control | 6.40 | 1 | 0.001 | |
| ANI vs. Control | 2.51 | 1 | 0.025 | |
| EA vs. Control | 1.60 | 1 | 0.073 | |
| PER vs. Control | 1.60 | 1 | 0.073 | |
| EUC vs. Control | 0.10 | 1 | 0.752 | |
| Pairwise comparisons among compounds (Liquid) | ANI vs. PER | 19.60 | 1 | <0.001 |
| CEO vs. PER | 10.76 | 1 | <0.001 | |
| ANI vs. CEO | 8.11 | 1 | <0.001 | |
| CIT vs. PER | 6.40 | 1 | 0.001 | |
| EA vs. PER | 2.51 | 1 | 0.025 | |
| CEO vs. EA | 1.60 | 1 | 0.206 | |
| ANI vs. EA | 0.90 | 1 | 0.343 | |
| CIT vs. CEO | 0.40 | 1 | 0.527 | |
| CIT vs. EA | 0.40 | 1 | 0.527 | |
| CIT vs. ANI | 0.10 | 1 | 0.752 | |
| Compounds vs. Control (LDPE 5%) | CEO vs. Control | 4.92 | 1 | 0.002 |
| ANI vs. Control | 1.60 | 1 | 0.206 | |
| EA vs. Control | 0.90 | 1 | 0.343 | |
| CIT vs. Control | 0.90 | 1 | 0.343 | |
| Compounds vs. Control (HDPE 3%) | ANI vs. Control | 14.40 | 1 | <0.001 |
| CEO vs. Control | 4.92 | 1 | 0.002 | |
| CIT vs. Control | 2.51 | 1 | 0.025 | |
| EA vs. Control | 0 | 1 | 1 | |
| Compounds vs. Control (HDPE 5%) | CEO vs. Control | 8.11 | 1 | <0.001 |
| EA vs. Control | 4.92 | 1 | 0.002 | |
| ANI vs. Control | 4.92 | 1 | 0.002 | |
| CIT vs. Control | 3.60 | 1 | 0.010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Pedro, L.; Sanchez, J.A. Natural Repellents as a Method of Preventing Ant Damage to Microirrigation Systems. Insects 2022, 13, 395. https://doi.org/10.3390/insects13040395
de Pedro L, Sanchez JA. Natural Repellents as a Method of Preventing Ant Damage to Microirrigation Systems. Insects. 2022; 13(4):395. https://doi.org/10.3390/insects13040395
Chicago/Turabian Stylede Pedro, Luis, and Juan Antonio Sanchez. 2022. "Natural Repellents as a Method of Preventing Ant Damage to Microirrigation Systems" Insects 13, no. 4: 395. https://doi.org/10.3390/insects13040395
APA Stylede Pedro, L., & Sanchez, J. A. (2022). Natural Repellents as a Method of Preventing Ant Damage to Microirrigation Systems. Insects, 13(4), 395. https://doi.org/10.3390/insects13040395