Impact and Persistence of Serratia marcescens in Tenebrio molitor Larvae and Feed under Optimal and Stressed Mass Rearing Conditions
Abstract
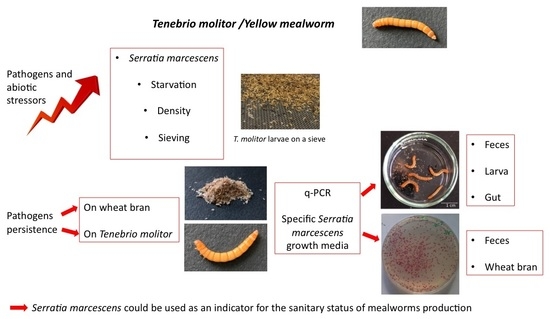
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tenebrio Molitor Rearing and Diet
2.2. Strain and Culture
2.3. Abiotic Stress Factors Combined with Sm Infection
2.4. Experimental Design
2.5. Variables Measured
2.6. Statistical Analyses: Stress Factors
2.7. In Vivo Experiment for Quantification of Sm by qPCR in Larva and Feces
2.8. Persistence of Sm in Inoculated Wheat Bran Feed
2.9. Monitoring of Sm Persistence in Tm Feces
3. Results
3.1. Impact of Stressors on Rearing Performances
- Larval mass relative growth and Feed Conversion Ratio (FCR)
- Stress impact on mortality
3.2. Detection of Sm by qPCR
Monitoring of Sm Persistence in Wheat Bran and Feces on Erythritol Selective Medium
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Huis, A. Potential of Insects as Food and Feed in Assuring Food Security. Annu. Rev. Èntomol. 2013, 58, 563–583. [Google Scholar] [CrossRef] [PubMed]
- Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A. Safety of dried yellow mealworm (Tenebrio molitor larva) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06343. [Google Scholar] [CrossRef] [PubMed]
- Cadinu, L.A.; Barra, P.; Torre, F.; Delogu, F.; Madau, F.A. Insect Rearing: Potential, Challenges, and Circularity. Sustainability 2020, 12, 4567. [Google Scholar] [CrossRef]
- Maciel-Vergara, G.; Jensen, A.; Lecocq, A.; Eilenberg, J. Diseases in edible insect rearing systems. J. Insects Food Feed 2021, 7, 621–638. [Google Scholar] [CrossRef]
- Maciel-Vergara, G.; Jensen, A.B.; Eilenberg, J. Cannibalism as a Possible Entry Route for Opportunistic Pathogenic Bacteria to Insect Hosts, Exemplified by Pseudomonas aeruginosa, a Pathogen of the Giant Mealworm Zophobas morio. Insects 2018, 9, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaver, D.K.; McFarlane, J. The effect of larval density on growth and development of Tenebrio molitor. J. Insect Physiol. 1990, 7, 531–536. [Google Scholar] [CrossRef]
- Jensen, A.N.; Hansen, S.H.; Baggesen, D.L. Salmonella Typhimurium Level in Mealworms (Tenebrio molitor) After Exposure to Contaminated Substrate. Front. Microbiol. 2020, 11, 1613. [Google Scholar] [CrossRef]
- Eilenberg, J.; Vlak, J.; Nielsen-Leroux, C.; Cappellozza, S.; Jensen, A.B. Diseases in insects produced for food and feed. J. Insects Food Feed 2015, 1, 87–102. [Google Scholar] [CrossRef] [Green Version]
- Vandeweyer, D.; De Smet, J.; van Looveren, N.; van Campenhout, L. Biological contaminants in insects as food and feed. J. Insects Food Feed 2021, 5, 807–822. [Google Scholar] [CrossRef]
- Urbanek, A.K.; Rybak, J.; Wróbel, M.; Leluk, K.; Mirończuk, A.M. A comprehensive assessment of microbiome diversity in Tenebrio molitor fed with polystyrene waste. Environ. Pollut. 2020, 262, 114281. [Google Scholar] [CrossRef]
- Cambon, M.C.; Ogier, J.C.J.; Lanois-Nouri, A.; Ferdy, J.-B.; Gaudriault, S. Changes in rearing conditions rapidly modify gut microbiota structure in Tenebrio molitor larvae. BioRxiv 2018. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.; Heo, A.; Park, Y.W.; Kim, Y.J.; Koh, H.; Park, W. Gut Microbiota of Tenebrio molitor and Their Response to Environmental Change. J. Microbiol. Biotechnol. 2014, 24, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Wynants, E.; Frooninckx, L.; van Miert, S.; Geeraerd, A.; Claes, J.; van Campenhout, L. Risks related to the presence of Salmonella sp. during rearing of mealworms (Tenebrio molitor) for food or feed: Survival in the substrate and transmission to the larvae. Food Control. 2019, 100, 227–234. [Google Scholar] [CrossRef]
- Pineda-Castellanos, M.L.; Rodríguez-Segura, Z.; Villalobos, F.J.; Hernández, L.; Lina, L.; Nuñez-Valdez, M.E. Pathogenicity of Isolates of Serratia marcescens towards Larvae of the Scarab Phyllophaga Blanchardi (Coleoptera). Pathogens 2015, 4, 210–228. [Google Scholar] [CrossRef] [Green Version]
- Khanna, A. Serratia marcescens-A Rare Opportunis tic Nosocomial Pathogen and Measures to Limit its Spread in Hospitalized Patients. J. Clin. Diagn. Res. 2013, 7, 243–246. [Google Scholar] [CrossRef]
- Steinhaus, E.A. Serratia marcescens Bizio as an insect pathogen. Hilgardia 1959, 28, 351–380. [Google Scholar] [CrossRef] [Green Version]
- Sikorowski, P.P.; Lawrence, A.M.; Inglis, G.D. Effects of Serratia marcescens on Rearing of the Tobacco Budworm (Lepidoptera: Noctuidae). Am. Entomol. 2001, 1, 51–60. [Google Scholar] [CrossRef]
- Slatten, B.H.; Larson, A. Mechanism of pathogenicity of Serratia marcescens. J. Invertebr. Pathol. 1967, 1, 78–81. [Google Scholar] [CrossRef]
- Raymann, K.; Coon, K.L.; Shaffer, Z.; Salisbury, S.; Moran, N.A. Pathogenicity of Serratia marcescens Strains in Honey Bees. mBio 2018, 5, e01649-18. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, F.K.P.; Brück, D.W.; Brück, W.M. Isolation of proteolytic bacteria from mealworm (Tenebrio molitor) exoskeletons to produce chitinous material. FEMS Microbiol. Lett. 2017, 364, fnx177. [Google Scholar] [CrossRef]
- Gomez, H.M.; Rivas, G.A.; Hernández-Quintero, A.; Hernández, A.G.; Guzmán, J.C.T.; Mendoza, H.L.; Contreras-Garduño, J. The occurrence of immune priming can be species-specific in entomopathogens. Microb. Pathog. 2018, 118, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Slotnick, I.J.; Dougherty, M. Erythritol as a Selective Substrate for the Growth of Serratia marcescens. Appl. Microbiol. 1972, 24, 292–293. [Google Scholar] [CrossRef] [PubMed]
- Lawson, J. Design and Analysis of Experiments with R, 1st ed.; Chapman and Hall/CRC: New York, NY, USA, 2015; pp. 94–105. [Google Scholar]
- Vigneron, A.; Jehan, C.; Rigaud, T.; Moret, Y. Immune Defenses of a Beneficial Pest: The Mealworm Beetle, Tenebrio molitor. Front. Physiol. 2019, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- Barnes, A.I.; Siva-Jothy, M.T. Density–dependent prophylaxis in the mealworm beetle Tenebrio molitor L. (Coleoptera: Tenebrionidae): Cuticular melanization is an indicator of investment in immunity. Proc. R. Soc. B Boil. Sci. 2000, 267, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Bjørge, J.D.; Overgaard, J.; Malte, H.; Gianotten, N.; Heckmann, L.-H.L. Role of temperature on growth and metabolic rate in the tenebrionid beetles Alphitobius diaperinus and Tenebrio molitor. J. Insect Physiol. 2018, 107, 89–96. [Google Scholar] [CrossRef]
- De Smet, J.; Wynants, E.; Cos, P.; van Campenhout, L. Microbial Community Dynamics during Rearing of Black Soldier Fly Larvae (Hermetia illucens) and Impact on Exploitation Potential. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef] [Green Version]
- Oppert, B.; Dowd, S.; Bouffard, P.; Li, L.; Conesa, A.; Lorenzen, M.; Toutges, M.; Marshall, J.; Huestis, D.; Fabrick, J.; et al. Transcriptome Profiling of the Intoxication Response of Tenebrio molitor Larvae to Bacillus thuringiensis Cry3Aa Protoxin. PLoS ONE 2012, 7, e34624. [Google Scholar] [CrossRef]







| One Cup/Treatment | Larval Density | Sieving | Starvation | Serratia |
|---|---|---|---|---|
| 1 | 0.4 g/cm2 | Manual | No | Water |
| 2 | 0.4 g/cm2 | Manual | Yes | Water |
| 3 | 0.4 g/cm2 | Mechanical | Yes | Water |
| 4 | 0.4 g/cm2 | Mechanical | Yes | Inoculum |
| 5 | 0.4 g/cm2 | Manual | No | Inoculum |
| 6 | 0.4 g/cm2 | Mechanical | No | Water |
| 7 | 0.4 g/cm2 | Manual | Yes | Inoculum |
| 8 | 0.4 g/cm2 | Mechanical | No | Inoculum |
| 9 | 0.8 g/cm2 | Manual | No | Water |
| 10 | 0.8 g/cm2 | Mechanical | No | Water |
| 11 | 0.8 g/cm2 | Mechanical | Yes | Water |
| 12 | 0.8 g/cm2 | Manual | Yes | Water |
| 13 | 0.8 g/cm2 | Mechanical | No | Inoculum |
| 14 | 0.8 g/cm2 | Mechanical | Yes | Inoculum |
| 15 | 0.8 g/cm2 | Manual | No | Inoculum |
| 16 | 0.8 g/cm2 | Manual | Yes | Inoculum |
| Parameter | Value | Standard Error | p-Value |
|---|---|---|---|
| Intercept | 8.11 | 0.12 | 7.43 × 10−23 |
| Day | −0.23 | 0.07 | 5.9 × 10−3 |
| Day2 | −0.03 | 8 × 10−3 | 1.5 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dupriez, F.; Rejasse, A.; Rios, A.; Lefebvre, T.; Nielsen-LeRoux, C. Impact and Persistence of Serratia marcescens in Tenebrio molitor Larvae and Feed under Optimal and Stressed Mass Rearing Conditions. Insects 2022, 13, 458. https://doi.org/10.3390/insects13050458
Dupriez F, Rejasse A, Rios A, Lefebvre T, Nielsen-LeRoux C. Impact and Persistence of Serratia marcescens in Tenebrio molitor Larvae and Feed under Optimal and Stressed Mass Rearing Conditions. Insects. 2022; 13(5):458. https://doi.org/10.3390/insects13050458
Chicago/Turabian StyleDupriez, Florent, Agnès Rejasse, Alfredo Rios, Thomas Lefebvre, and Christina Nielsen-LeRoux. 2022. "Impact and Persistence of Serratia marcescens in Tenebrio molitor Larvae and Feed under Optimal and Stressed Mass Rearing Conditions" Insects 13, no. 5: 458. https://doi.org/10.3390/insects13050458
APA StyleDupriez, F., Rejasse, A., Rios, A., Lefebvre, T., & Nielsen-LeRoux, C. (2022). Impact and Persistence of Serratia marcescens in Tenebrio molitor Larvae and Feed under Optimal and Stressed Mass Rearing Conditions. Insects, 13(5), 458. https://doi.org/10.3390/insects13050458