Chili Pepper Jojutla Morelos (Capsicum annuum L.), CJ-2018: A Variety Resistant to Bactericera cockerelli
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
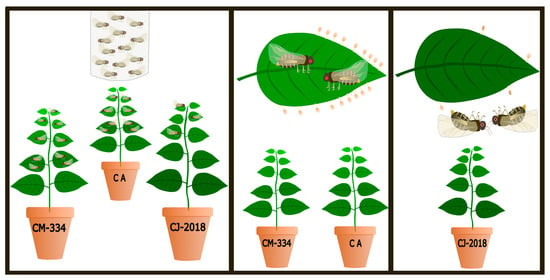
2.1. Biological Material
2.2. Oviposition Preference
2.2.1. Choice Assays
2.2.2. Non-Choice Assays
2.3. Growth and Survival of Bactericera cockerelli
2.4. Statistical Analysis
3. Results
3.1. Oviposition Preference, Choice, and Non-Choice Assays for Six Days
3.2. Oviposition Preference, Choice, and Non-Choice Assays at Different Times
3.3. Development and Survival of Bactericera cockerelli
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Servicio de Información Agroalimentaria y Pesquera (SIAP). Panorama Agroalimentario 2021. Conectando Conocimiento Ancentral y Moderno Para Lograr La Autosuficiencia Agroalimentaria. Available online: https://nube.siap.gob.mx/gobmx_publicaciones_siap/pag/2021/Panorama-Agroalimentario-2021 (accessed on 7 June 2022).
- Hansen, A.K.; Trumble, J.T.; Stouthamer, R.; Paine, T.D. A New Huanglongbing Species, “Candidatus Liberibacter Psyllaurous”, Found to Infect Tomato and Potato, Is Vectored by the Psyllid Bactericera Cockerelli (Sulc). Appl. Environ. Microbiol. 2008, 74, 5862–5865. [Google Scholar] [CrossRef] [PubMed]
- Liefting, L.W.; Perez-Egusquiza, Z.C.; Clover, G.R.G.; Anderson, J.A.D. A New “Candidatus Liberibacter” Species in Solanum Tuberosum in New Zealand. Plant Dis. 2008, 92, 1474. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Tapia, M.; Rojas-Martínez, R.I.; Zavaleta-Mejía, E.; Hernández-Deheza, M.G.; Carrillo-Salazar, J.A.; And, R.-A.A.; Ochoa-Martínez, D.L. Aetiology of Chili Pepper Variegation from Yurécuaro, México. J. Plant Pathol. 2011, 93, 331–335. [Google Scholar]
- Velásquez-Valle, R.; Reveles-Torres, L.R.; Mena-Covarrubias, J.; Salas-Muñoz, S.; Mauricio-Castillo, J.A. Outbreak of Candidatus Liberibacter Solanacearum in Dried Chile Pepper in Durango, Mexico. Agrofaz 2014, 14, 93–104. [Google Scholar]
- Dávila Medina, M.D.; Cerna Chávez, E.; Aguirre Uribe, L.A.; García Martínez, O.; Ochoa Fuentes, Y.M.; Gallegos Morales, G.; Landeros Flores, J. Susceptibilidad y Mecanismos de Resistencia a Insecticidas En Bactericera Cockerelli (Sulc.) En Coahuila, México. Rev. Mex. Cienc. Agríc. 2012, 3, 1145–1155. [Google Scholar] [CrossRef]
- Cerna, E.; Ochoa, Y.; Aguirre, L.A.; Flores, M.; Landeros, J. Determinación de La Resistencia a Insecticidas En Cuatro Poblaciones Del Psílido de La Papa Bactericera Cockerelli (Sulc) (Hemiptera: Triozidae). Rev. Int. Bot. Exp. 2013, 9457, 63–68. [Google Scholar]
- Cerna-Chávez, E.; Bautista, O.H.; Flores, J.L.; Uribe, L.A.; Fuentes, Y.M.O. Insecticide-Resistance Ratios of Three Populations of Bactericera Cockerelli (Hemiptera: Psylloidea: Triozidae) in Regions of Northern Mexico. Fla. Entomol. 2015, 98, 950–953. [Google Scholar] [CrossRef]
- Smith, C.M. Plant Resistance to Arthropods; Springer: Dordrecht, The Netherlands, 2005; ISBN 2013206534. [Google Scholar]
- Koch, K.G.; Chapman, K.; Louis, J.; Heng-Moss, T.; Sarath, G. Plant Tolerance: A Unique Approach to Control Hemipteran Pests. Front. Plant Sci. 2016, 7, 1–12. [Google Scholar] [CrossRef]
- Mitchell, C.; Brennan, R.M.; Graham, J.; Karley, A.J. Plant Defense against Herbivorous Pests: Exploiting Resistance and Tolerance Traits for Sustainable Crop Protection. Front. Plant Sci. 2016, 7, 1363. [Google Scholar] [CrossRef]
- Kraft, K.H.; Brown, C.H.; Nabhan, G.P.; Luedeling, E.; De Jesús Luna Ruiz, J.; D’Eeckenbrugge, G.C.; Hijmans, R.J.; Gepts, P. Multiple Lines of Evidence for the Origin of Domesticated Chili Pepper, Capsicum Annuum, in Mexico. Proc. Natl. Acad. Sci. USA 2014, 111, 6165–6170. [Google Scholar] [CrossRef]
- Villar-Luna, E.; Rojas-Martínez, R.I.; Reyes-Trejo, B.; Gómez-Rodríguez, O.; Zavaleta-Mejía, E. Mevalonate Pathway Genes Expressed in Chilli CM334 Inoculated with Phytophthora Capsici and Infected by Nacobbus Aberrans and Meloidogyne Enterolobii. Eur. J. Plant Pathol. 2017, 148, 867–881. [Google Scholar] [CrossRef]
- Boiteux, L.S.; Cupertino, F.P.; Silva, C.; Dusi, A.N.; Monte-Neshich, D.C.; Van Der Vlugt, R.A.; Fonseca, M.E. Resistance to Potato Virus Y (Pathotype 1–2) in Capsicum Annuum and Capsicum Chinense Is Controlled by Two Independent Major Genes. Euphytica 1996, 87, 53–58. [Google Scholar] [CrossRef]
- Janzac, B.; Fabre, M.F.; Palloix, A.; Moury, B. Phenotype and Spectrum of Action of the Pvr4 Resistance in Pepper against Potyviruses, and Selection for Virulent Variants. Plant Pathol. 2009, 58, 443–449. [Google Scholar] [CrossRef]
- Djian-Caporalino, C.; Pijarowski, L.; Januel, A.; Lefebvre, V.; Daubèze, A.; Palloix, A.; Dalmasso, A.; Abad, P. Spectrum of Resistance to Root-Knot Nematodes and Inheritance of Heat-Stable Resistance in in Pepper (Capsicum annuum L.). Theor. Appl. Genet. 1999, 99, 496–502. [Google Scholar] [CrossRef]
- Djian-Caporalino, C.; Fazari, A.; Arguel, M.J.; Vernie, T.; Vande Casteele, C.; Faure, I.; Brunoud, G.; Pijarowski, L.; Palloix, A.; Lefebvre, V.; et al. Root-Knot Nematode (Meloidogyne spp.) Me Resistance Genes in Pepper (Capsicum annuum L.) Are Clustered on the P9 Chromosome. Theor. Appl. Genet. 2007, 114, 473–486. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- do Valle, G.E.; Lourenção, A.L.; Pinheiro, J.B. Adult Attractiveness and Oviposition Preference of Bemisia Tabaci Biotype B in Soybean Genotypes with Different Trichome Density. J. Pest Sci. 2012, 85, 431–442. [Google Scholar] [CrossRef]
- Baldin, E.L.L.; Cruz, P.L.; Morando, R.; Silva, I.F.; Bentivenha, J.P.F.; Tozin, L.R.S.; Rodrigues, T.M. Characterization of Antixenosis in Soybean Genotypes to Bemisia Tabaci (Hemiptera: Aleyrodidae) Biotype B. J. Econ. Entomol. 2017, 110, 1869–1876. [Google Scholar] [CrossRef]
- Schlick-Souza, E.C.; Baldin, E.L.L.; Morando, R.; Lourenção, A.L. Antixenosis to Chrysodeixis Includens (Lepidoptera: Noctuidae) among Soybean Genotypes. Bragantia 2018, 77, 124–133. [Google Scholar] [CrossRef]
- Ongaratto, S.; Silveira, C.M.; Santos, M.C.; Gorri, J.E.R.; Sartori, M.M.P.; Hunt, T.E.; Lourenção, A.L.; Baldin, E.L.L. Resistance of Soybean Genotypes to Anticarsia Gemmatalis (Lepidoptera: Erebidae): Antixenosis and Antibiosis Characterization. J. Econ. Entomol. 2021, 114, 2571–2580. [Google Scholar] [CrossRef]
- Firdaus, S.; Van Heusden, A.; Harpenas, A.; Supena, E.D.J.; Visser, R.G.F.; Vosman, B. Identification of Silverleaf Whitefly Resistance in Pepper. Plant Breed. 2011, 130, 708–714. [Google Scholar] [CrossRef]
- Da Silva, A.G.; Boica Junior, A.L.; Farias, P.R.S.; Rodrigues, N.E.L.; De Souza, B.H.S.; Bottega, D.B.; Chiorato, A.F. Non-Preference for Oviposition and Antibiosis in Bean Cultivars to Bemisia Tabaci Biotype B (Hemiptera: Aleyrodidae). Rev. Colomb. Entomol. 2014, 40, 7–14. [Google Scholar]
- Pantoja, K.F.C.; Rocha, K.C.G.; Melo, A.M.T.; Marubayashi, J.M.; Baldin, E.L.L.; Bentivenha, J.P.F.; Gioria, R.; Kobori, R.F.; Pavan, M.A.; Krause-Sakate, R. Identification of Capsicum Accessions Tolerant to Tomato Severe Rugose Virus and Resistant to Bemisia Tabaci Middle East-Asia Minor 1 (MEAM1). Trop. Plant Pathol. 2018, 43, 138–145. [Google Scholar] [CrossRef]
- Hernández-Alvarado, L.A.; Ruiz-Sánchez, E.; Latournerie-Moreno, L.; Garruña-Hernández, R.; González-Mendoza, D.; Chan-Cupul, W. Resistance of Capsicum Annuum Genotypes to Bemisia Tabaci and Influence of Plant Leaf Traits. Rev. Fitotec. Mex. 2019, 42, 251–257. [Google Scholar] [CrossRef]
- Syamsudin, T.S.; Kirana, R.; Karjadi, A.K.; Faizal, A. Characteristics of Chili (Capsicum Annuum L.) That Are Resistant and Susceptible to Oriental Fruit Fly (Bactrocera dorsalis Hendel) Infestation. Horticulturae 2022, 8, 314. [Google Scholar] [CrossRef]
- Lee, J.H.J.; Awika, H.O.; Jayaprakasha, G.K.; Avila, C.A.; Crosby, K.M.; Patil, B.S. Tomato Metabolic Changes in Response to Tomato-Potato Psyllid (Bactericera Cockerelli) and Its Vectored Pathogen Candidatus Liberibacter Solanacearum. Plants 2020, 9, 1154. [Google Scholar]
- Chen, R.; Li, H.; Zhang, L.; Zhang, J.; Xiao, J.; Ye, Z. CaMi, a Root-Knot Nematode Resistance Gene from Hot Pepper (Capsium Annuum L.) Confers Nematode Resistance in Tomato. Plant Cell Rep. 2007, 26, 895–905. [Google Scholar] [CrossRef]
- Gilbert, J.; McGuire, D. Inheritance of Resistance to Severe Root-Knot from Meloidogyne Incognita in Commercial-Type Tomatoes. Proc. Am. Soc. Hortic. Sci. 1956, 68, 437–442. [Google Scholar]
- Rossi, M.; Goggin, F.L.; Milligan, S.B.; Kaloshian, I.; Ullman, D.E.; Williamson, V.M. The Nematode Resistance Gene Mi of Tomato Confers Resistance against the Potato Aphid. Proc. Natl. Acad. Sci. USA 1998, 95, 9750–9754. [Google Scholar] [CrossRef]
- Vos, P.; Simons, G.; Jesse, T.; Wijbrandi, J.; Heinen, L.; Hogers, R.; Frijters, A.; Groenendijk, J.; Diergaarde, P.; Reijans, M.; et al. The Tomato Mi-1 Gene Confers Resistance to Both Root-Knot Nematodes and Potato Aphids. Nat. Biotechnol. 1998, 16, 1365–1369. [Google Scholar] [CrossRef]
- Nombela, G.; Williamson, V.M.; Muñiz, M. The Root-Knot Nematode Resistance Gene Mi-1.2 of Tomato Is Responsible for Resistance against the Whitefly Bemisia Tabaci. Mol. Plant-Microbe Interact. 2003, 16, 645–649. [Google Scholar] [CrossRef]
- Casteel, C.L.; Walling, L.L.; Paine, T.D. Behavior and Biology of the Tomato Psyllid, Bactericerca Cockerelli, in Response to the Mi-1.2 Gene. Entomol. Exp. Appl. 2006, 121, 67–72. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Mikagi, E.; Mekuria, D.B.; Borua, A.D.; Tebayashi, S.I.; Kim, C.S. Ovipositional Deterrent on Mature Stage of Sweet Pepper, Capsicum Annuum, against Liriomyza Trifolii (Burgess). Z. Naturforsch. C J. Biosci. 2005, 60, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Quiroz, A.; Pettersson, J.; Pickett, J.A.; Wadhams, L.J.; Niemeyer, H.M. Semiochemicals Mediating Spacing Behavior of Bird Cherry-Oat Aphid. J. Chem. Ecol. 1997, 23, 2599–2607. [Google Scholar] [CrossRef]
- da Costa, J.G.; Pires, E.V.; Riffel, A.; Birkett, M.A.; Bleicher, E.; Sant’Ana, A.E.G. Differential Preference of Capsicum Spp. Cultivars by Aphis Gossypii Is Conferred by Variation in Volatile Semiochemistry. Euphytica 2011, 177, 299–307. [Google Scholar] [CrossRef]
- Bleeker, P.M.; Diergaarde, P.J.; Ament, K.; Guerra, J.; Weidner, M.; Schütz, S.; de Both, M.T.J.; Haring, M.A.; Schuurink, R.C. The Role of Specific Tomato Volatiles in Tomato-Whitefly Interaction. Plant Physiol. 2009, 151, 925–935. [Google Scholar] [CrossRef]
- Levy, J.; Tamborindeguy, C. Solanum Habrochaites, a Potential Source of Resistance against Bactericera Cockerelli (Hemiptera: Triozidae) and “Candidatus Liberibacter Solanacearum”. J. Econ. Entomol. 2014, 107, 1187–1193. [Google Scholar] [CrossRef]
- Avila, C.A.; Marconi, T.G.; Viloria, Z.; Kurpis, J.; Del Rio, S.Y. Bactericera Cockerelli Resistance in the Wild Tomato Solanum Habrochaites Is Polygenic and Influenced by the Presence of Candidatus Liberibacter Solanacearum. Sci. Rep. 2019, 9, 14031. [Google Scholar] [CrossRef]
- Mayo-Hernández, J.; Flores-Olivas, A.; Valenzuela-Soto, J.; Rodríguez-Pagaza, Y.; Vega-Chávez, J.; Hernández-Castillo, F.; Aguirre-Uribe, L. Bactericera Cockerelli Sulc Oviposition Preference and Development on Three Tomato Varieties. Southwest. Entomol. 2018, 43, 905–910. [Google Scholar] [CrossRef]
- Mayo-Hernández, J.; Ramírez-Chávez, E.; Molina-Torres, J.; Guillén-Cisneros, M.D.L.; Rodríguez-Herrera, R.; Hernández-Castillo, F.; Flores-Olivas, A.; Valenzuela-Soto, J.H. Effects of Bactericera cockerelli Herbivory on Volatile Emissions of Three Varieties of Solanum lycopersicum. Plants 2019, 8, 509. [Google Scholar] [CrossRef]
- Maharijaya, A.; Vosman, B.; Steenhuis-Broers, G.; Harpenas, A.; Purwito, A.; Visser, R.G.F.; Voorrips, R.E. Screening of Pepper Accessions for Resistance against Two Thrips Species (Frankliniella occidentalis and Thrips parvispinus). Euphytica 2011, 177, 401–410. [Google Scholar] [CrossRef]
- Maharijaya, A.; Vosman, B.; Pelgrom, K.; Wahyuni, Y.; de Vos, R.C.H.; Voorrips, R.E. Genetic Variation in Phytochemicals in Leaves of Pepper (Capsicum) in Relation to Thrips Resistance. Arthropod-Plant Interact. 2019, 13, 1–9. [Google Scholar] [CrossRef]
- Li, Z.; Kund, G.; De Jong, D.M.; Feng, X.; Mutschler, M.A.; Trumble, J.T. Effects of High-Level Acylsugar-Producing Tomato Lines on the Development of Tomato Psyllids (Hemiptera: Triozidae). J. Econ. Entomol. 2019, 112, 1926–1931. [Google Scholar] [CrossRef]
- Butler, C.D.; Gonzalez, B.; Manjunath, K.L.; Lee, R.F.; Novy, R.G.; Miller, J.C.; Trumble, J.T. Behavioral Responses of Adult Potato Psyllid, Bactericera Cockerelli (Hemiptera: Triozidae), to Potato Germplasm and Transmission of Candidatus Liberibacter Psyllaurous. Crop Prot. 2011, 30, 1233–1238. [Google Scholar] [CrossRef]
- Diaz-Montano, J.; Vindiola, B.G.; Drew, N.; Novy, R.G.; Miller, J.C.; Trumble, J.T. Resistance of Selected Potato Genotypes to the Potato Psyllid (Hemiptera: Triozidae). Am. J. Potato Res. 2014, 91, 363–367. [Google Scholar] [CrossRef]
- Cooper, W.R.; Bamberg, J.B. Variation in Bactericera Cockerelli (Hemiptera: Triozidae) Oviposition, Survival, and Development on Solanum Bulbocastanum Germplasm. Am. J. Potato Res. 2014, 91, 532–537. [Google Scholar] [CrossRef]
- Fife, A.N.; Cruzado, K.; Rashed, A.; Novy, R.G.; Wenninger, E.J. Potato Psyllid (Hemiptera: Triozidae) Behavior on Three Potato Genotypes with Tolerance to “Candidatus Liberibacter Solanacearum”. J. Insect Sci. 2020, 20, 15. [Google Scholar] [CrossRef]


| Variety of Chili | Number of Eggs | |
|---|---|---|
| Choice | Non-Choice | |
| ‘Árbol’ | 358.60 ± 29.64 b | 333.80 ± 46.41 a |
| ‘Criollo de Morelos, CM-334’ | 718.80 ± 50.72 a | 292.10 ± 35.13 b |
| ‘Criollo de Jojutla, CJ-2018’ | 28.40 ± 7.22 c | 67.00 ± 9.97 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Valenzuela, M.; Rojas-Martínez, R.I.; Zúñiga-Mayo, V.M. Chili Pepper Jojutla Morelos (Capsicum annuum L.), CJ-2018: A Variety Resistant to Bactericera cockerelli. Insects 2022, 13, 742. https://doi.org/10.3390/insects13080742
Silva-Valenzuela M, Rojas-Martínez RI, Zúñiga-Mayo VM. Chili Pepper Jojutla Morelos (Capsicum annuum L.), CJ-2018: A Variety Resistant to Bactericera cockerelli. Insects. 2022; 13(8):742. https://doi.org/10.3390/insects13080742
Chicago/Turabian StyleSilva-Valenzuela, Manuel, Reyna Isabel Rojas-Martínez, and Victor M. Zúñiga-Mayo. 2022. "Chili Pepper Jojutla Morelos (Capsicum annuum L.), CJ-2018: A Variety Resistant to Bactericera cockerelli" Insects 13, no. 8: 742. https://doi.org/10.3390/insects13080742
APA StyleSilva-Valenzuela, M., Rojas-Martínez, R. I., & Zúñiga-Mayo, V. M. (2022). Chili Pepper Jojutla Morelos (Capsicum annuum L.), CJ-2018: A Variety Resistant to Bactericera cockerelli. Insects, 13(8), 742. https://doi.org/10.3390/insects13080742